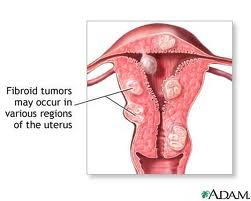
 Uterine fibroids are the most common, non-cancerous tumors in women of childbearing age. The fibroids are made of muscle cells and other tissues that grow within and around the wall of the uterus. See the diagram that shows where uterine fibroids may grow.
Uterine fibroids are the most common, non-cancerous tumors in women of childbearing age. The fibroids are made of muscle cells and other tissues that grow within and around the wall of the uterus. See the diagram that shows where uterine fibroids may grow.
There are several risk factors for uterine fibroids:
- African American woman are at three- to five-times greater risk than white women for fibroids.
- Women who are overweight or obese for their height are at greater risk.
- Women who have given birth are a lower risk.
Many women with uterine fibroids have no symptoms. Symptoms of uterine fibroids can include:
- Heavy or painful periods, or bleeding between periods
- Feeling “full” in the lower abdomen
- Urinating often
- Pain during sex
- Lower back pain
- Reproductive problems, such as infertility, multiple miscarriages, or early labor
Most women with fibroids do not have problems with fertility and can get pregnant. However, some women with fibroids may not be able to get pregnant naturally, but advances in treatments for infertility may help some of these women get pregnant.
If you have uterine fibroids, but show no symptoms, you many not need any treatment. Women who have pain and other symptoms might benefit from treatment which includes medication and/or surgery. Medications can offer relief from the symptoms of fibroids and even slow or stop their growth. But, once you stop taking the medicine, the fibroids often grow back. There are several types of fibroid surgery:
- Myomectomy – Removes only the fibroids and leaves the healthy areas of the uterus in place
- Uterine Artery Embolization (UAE) – Cuts off the blood supply to the uterus and fibroids, making them shrink
- Hysterectomy - A more major procedure that removes the uterus; this type of surgery is the only sure way to cure fibroids.
For more details about treatments for fibroids, see the free booklet from the NICHD.
In addition, if you have fibroids and need support, the Fibroids Project is a one-stop shop for women with uterine fibroids created by a women with who has been there.

 Today Is the Great American Smokeout--November 18--time to Quit!
Today Is the Great American Smokeout--November 18--time to Quit! The following blog was posted on the
The following blog was posted on the  As an Institute that promotes sex and gender research, here is another blog of particular interest to the male side of the equation!
As an Institute that promotes sex and gender research, here is another blog of particular interest to the male side of the equation! Alzheimer's disease affects twice as many women as it does men, according to a new report that portrays women as being "under siege" by the dreaded condition.
Alzheimer's disease affects twice as many women as it does men, according to a new report that portrays women as being "under siege" by the dreaded condition.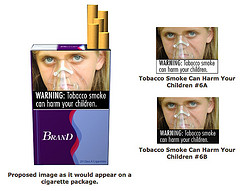 Federal regulators are testing the waters with the first proposed changes to cigarette packaging and advertisements in more than 25 years—bold health warnings with color images that show the tragic consequences of smoking.
Federal regulators are testing the waters with the first proposed changes to cigarette packaging and advertisements in more than 25 years—bold health warnings with color images that show the tragic consequences of smoking. To view the warning labels, go to:
To view the warning labels, go to:  In celebration of National Diabetes Awareness Month, the
In celebration of National Diabetes Awareness Month, the  The first research from the
The first research from the  Between 2000 and 2007, the death rate of men treated in hospitals for stroke tumbled by 29 percent compared to a 24 percent decline for women, according to the latest News and Numbers from the Agency for Healthcare Research and Quality (AHRQ).
Between 2000 and 2007, the death rate of men treated in hospitals for stroke tumbled by 29 percent compared to a 24 percent decline for women, according to the latest News and Numbers from the Agency for Healthcare Research and Quality (AHRQ).