 This June, the Food and Drug Administration (FDA) will release a revision to prescription guidelines--the first since 1979. These new guidelines will provide up-to-date and specific information to doctors about the risks and benefits of medication that pregnant women may need to control other conditions. The rule of thumb over the years has just been to tough it out and not take any medicines that may (or may not) hurt the mother or the fetus. Research in this area is limited because pregnant women are excluded from most drug trials. Dr. Katherine Wisner, WHRI Leadership Council Member and expert on mood disorders at Northwestern U, "Pregnant women get sick and sex women get pregnant. But somehow we have created this myth of the medication-free pregnancy." We've all heard stories about pregnant women who have serious depression and stop their meds---harming themselves or their baby because their condition is out of control.
This June, the Food and Drug Administration (FDA) will release a revision to prescription guidelines--the first since 1979. These new guidelines will provide up-to-date and specific information to doctors about the risks and benefits of medication that pregnant women may need to control other conditions. The rule of thumb over the years has just been to tough it out and not take any medicines that may (or may not) hurt the mother or the fetus. Research in this area is limited because pregnant women are excluded from most drug trials. Dr. Katherine Wisner, WHRI Leadership Council Member and expert on mood disorders at Northwestern U, "Pregnant women get sick and sex women get pregnant. But somehow we have created this myth of the medication-free pregnancy." We've all heard stories about pregnant women who have serious depression and stop their meds---harming themselves or their baby because their condition is out of control.
The old system used a scoring system of A, B, C, D, and X with ' X" being the most dangerous. The new system will have three components:
- Information on dosing and risks to the fetus
- Known risks about the drug's impact on breast feeding (e.g. will it concentrate in the milk)
- Drug's impact on fertility.
According to the CDC, about 90% of pregnant women are on at least one prescribed or OTC medication. Providing doctors more labeling information with help them determine safe options for treatment and help women have a healthier pregnancy..
Read more in the Chicago Tribune.


 Some women need to take medicines during pregnancy for health problems like diabetes, depression, morning sickness or seizures. Always talk with your doctor, nurse, or pharmacist before taking any medicines, vitamins or herbs. Don't stop taking your prescription medicines unless your health care provider says that it is OK.
Some women need to take medicines during pregnancy for health problems like diabetes, depression, morning sickness or seizures. Always talk with your doctor, nurse, or pharmacist before taking any medicines, vitamins or herbs. Don't stop taking your prescription medicines unless your health care provider says that it is OK. As the state with the highest rates of fetal alcohol syndrome (FAS) in the U.S., Alaska is introducing a new campaign aimed at preventing pregnant women from drinking, the Anchorage Daily News reported. Starting in December, pregnancy tests will be placed in the bathrooms of 20 bars and restaurants across the state.
As the state with the highest rates of fetal alcohol syndrome (FAS) in the U.S., Alaska is introducing a new campaign aimed at preventing pregnant women from drinking, the Anchorage Daily News reported. Starting in December, pregnancy tests will be placed in the bathrooms of 20 bars and restaurants across the state. Depression, especially in pregnancy, is a sensitive subject. It impacts the woman, her child and her family and it affects between 14 and 23% women during pregnancy. Because of hormonal changes during pregnancy, a woman may not realize she is suffering from depression. A new, comprehensive guide about this condition that discusses symptoms and treatment to help women and their family members understand and cope with this issue is now available from the a site called PsychGuides. For a helpful resource visit
Depression, especially in pregnancy, is a sensitive subject. It impacts the woman, her child and her family and it affects between 14 and 23% women during pregnancy. Because of hormonal changes during pregnancy, a woman may not realize she is suffering from depression. A new, comprehensive guide about this condition that discusses symptoms and treatment to help women and their family members understand and cope with this issue is now available from the a site called PsychGuides. For a helpful resource visit 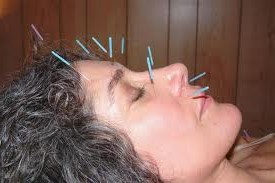 Acupuncture has been used in Eastern countries to address women's health issues but is not readily adopted in the U.S. especially by the medical establishment. A new study reports preliminary data indicating that acupuncture may improve menstrual health and overcome delays in becoming pregnant. There are experimental data indicating that acupuncture can influence female reproductive functioning, although the actual mechanisms involved are not yet clarified. Acupuncture is a complex intervention yet the evaluation of acupuncture research designs and outcome measures expect a level of commensurability difficult to achieve in complex interventions. A focus on effectiveness rather than efficacy may be a solution. Further research is needed that includes the rich traditions of acupuncture practice and the rigorous methods of evidence-based research.
Acupuncture has been used in Eastern countries to address women's health issues but is not readily adopted in the U.S. especially by the medical establishment. A new study reports preliminary data indicating that acupuncture may improve menstrual health and overcome delays in becoming pregnant. There are experimental data indicating that acupuncture can influence female reproductive functioning, although the actual mechanisms involved are not yet clarified. Acupuncture is a complex intervention yet the evaluation of acupuncture research designs and outcome measures expect a level of commensurability difficult to achieve in complex interventions. A focus on effectiveness rather than efficacy may be a solution. Further research is needed that includes the rich traditions of acupuncture practice and the rigorous methods of evidence-based research. A
A  Swedish doctors are attempting an innovative surgery to give womb-less women the opportunity to give birth to their own children. Nine women in Sweden have received womb transplants and doctors intend to help these women (through in-vitro fertilization) become pregnant and carry their own children. Each of the nine patients was either born without a uterus or had it removed due to cervical cancer. This is the fist major experiment to test the possibility of live, biological births in womb transplant patients.
Swedish doctors are attempting an innovative surgery to give womb-less women the opportunity to give birth to their own children. Nine women in Sweden have received womb transplants and doctors intend to help these women (through in-vitro fertilization) become pregnant and carry their own children. Each of the nine patients was either born without a uterus or had it removed due to cervical cancer. This is the fist major experiment to test the possibility of live, biological births in womb transplant patients. New research shows that women with high blood pressure during pregnancy may be at higher risk of having troublesome menopausal symptoms in the future. A research study from the Netherlands examined the relationship between hypertensive diseases and hot flashes and night sweats.
New research shows that women with high blood pressure during pregnancy may be at higher risk of having troublesome menopausal symptoms in the future. A research study from the Netherlands examined the relationship between hypertensive diseases and hot flashes and night sweats. The obstetrical and gynecological manifestation of Crohn's disease is particularly challenging for young women and demands special attention, according to an
The obstetrical and gynecological manifestation of Crohn's disease is particularly challenging for young women and demands special attention, according to an