 A new book, The Birth of the Pill, by Jonathan Eig tracks the involvement of four individuals who were key crusaders in the advent of readily available contraception that women controlled! For readers who were not around for the sexual revolution of the late 1960s, here are some factoids that you might find interesting:
A new book, The Birth of the Pill, by Jonathan Eig tracks the involvement of four individuals who were key crusaders in the advent of readily available contraception that women controlled! For readers who were not around for the sexual revolution of the late 1960s, here are some factoids that you might find interesting:
- Four key players are featured in the book: Margaret Sanger, feminist, and co-founder Planned parenthood; Gregory Pincus, biologist who was fired by Harvard for his controversial research on in vitro fertilization and is considered the "inventor" of the Pill ; John Rock, a Catholic, ran clinical trials on the pill, and served as an intermediary with his Church; and Katherine McCormick, an heiress who funded the research on the pill.
- Early on, due to Rock's intervention, the Vatican almost supported the pill as a natural form of "rhythm"...but in the end did not!
- One of the pre-FDA approval users of the pill was Sue Dixon whose father was Jack Searle the CEO of G.D. Searle and Company--the company that eventually marketed the pill.
- Early clinical trials for the pill were conducted in Puerto Rico where, at the time, regulations for consent and other ethical issues was far less stringent than they are now.
- Early versions of the pill were stronger than necessary because scientists wanted to be sure it was 100% effective. Today, the dosages has been scaled down considerably reducing risks and adverse effects.
- David Wagner, a product engineer at Illinois Tool Works, Inc. designed a circular pill dispenser after his wife complained of forgetting to take the pill everyday. His design was rejected by Searle but picked up by Ortho who was preparing to release their own version on the pill called "dialpak". This dispenser design helps distinguish the "pill" in a unique way that still exists today!
- The pill was initially FDA approved as a treatment for menstrual disorders, giving its manufacturer a "gentle" release into the market.

 A new
A new  The U.S. Food and Drug Administration today allowed marketing of the inFlow Intraurethral Valve-Pump, a replaceable urinary prosthesis for use in female adults who cannot contract the muscles necessary to push urine out of the bladder (impaired detrusor contractility or IDC).
The U.S. Food and Drug Administration today allowed marketing of the inFlow Intraurethral Valve-Pump, a replaceable urinary prosthesis for use in female adults who cannot contract the muscles necessary to push urine out of the bladder (impaired detrusor contractility or IDC). Today, NIH released a Guide Notice (NOT-OD-15-012) announcing that the response date for the Request for Information “
Today, NIH released a Guide Notice (NOT-OD-15-012) announcing that the response date for the Request for Information “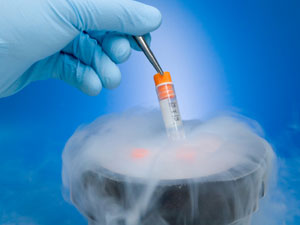 In a bold announcement today, Apple and Facebook now will offer health coverage for their women employees to freeze their eggs. Egg freezing may enable women to protect and preserve their fertility—and with the steep price of $10,000+, this coverage may be seen as a significant investment in family planning, while others may see this as concerning. Climbing the corporate ladder while raising a family can be a significant barrier for many women and the health coverage to freeze one’s eggs can provide women with the choice and freedom to devote time to work and to one’s family. However, some argue this potentially pushing women to focus on their careers as primary and family as secondary.
In a bold announcement today, Apple and Facebook now will offer health coverage for their women employees to freeze their eggs. Egg freezing may enable women to protect and preserve their fertility—and with the steep price of $10,000+, this coverage may be seen as a significant investment in family planning, while others may see this as concerning. Climbing the corporate ladder while raising a family can be a significant barrier for many women and the health coverage to freeze one’s eggs can provide women with the choice and freedom to devote time to work and to one’s family. However, some argue this potentially pushing women to focus on their careers as primary and family as secondary. Perimenopause is the time when a women naturally starts having menopausal symptoms. This natural change usually lasts about a year and is often referred to as the 'menopause transition'. At this time, fertility declines but a woman may still get pregnant, and effective birth control should be used if she does not want to have a mid-life baby. Generally, after a year of no menses, a woman can be considered infertile and menopausal.
Perimenopause is the time when a women naturally starts having menopausal symptoms. This natural change usually lasts about a year and is often referred to as the 'menopause transition'. At this time, fertility declines but a woman may still get pregnant, and effective birth control should be used if she does not want to have a mid-life baby. Generally, after a year of no menses, a woman can be considered infertile and menopausal. Uterine fibroids are the most common noncancerous tumors in women of childbearing age and the second most common reason these women undergo surgery. Uterine fibroids can lead to significant pain, bleeding, and fertility problems. Treatment options include watchful waiting; treatment with drugs or hormones, embolization, or ultrasound; and invasive procedures such as partial or total hysterectomy. However, there is little evidence about the effectiveness of these therapies or their outcomes, including fibroid reoccurrence and women’s ability to have children.
Uterine fibroids are the most common noncancerous tumors in women of childbearing age and the second most common reason these women undergo surgery. Uterine fibroids can lead to significant pain, bleeding, and fertility problems. Treatment options include watchful waiting; treatment with drugs or hormones, embolization, or ultrasound; and invasive procedures such as partial or total hysterectomy. However, there is little evidence about the effectiveness of these therapies or their outcomes, including fibroid reoccurrence and women’s ability to have children. Last Spring,
Last Spring,  Who doesn't like something sweet like a cupcake? But how much is too much? Our bodies need one type of sugar: glucose! It's an important source of fuel for the body.
Who doesn't like something sweet like a cupcake? But how much is too much? Our bodies need one type of sugar: glucose! It's an important source of fuel for the body. October is Breast Cancer Awareness Month, and, in the city of Chicago there are many ways to get involved. Classes, races,
October is Breast Cancer Awareness Month, and, in the city of Chicago there are many ways to get involved. Classes, races,