The following essay by Institute Director Woodruff was published this month in
Science2034.
In 2007, I was asked by the Medical Sciences Graduate Students Association at the University of Calgary to participate in a symposium called “Pushing the Boundaries – Advances That Will Change the World in 20 Years.” My presentation topic was oncofertility—a word I had just coined to describe the intersection of two disciplines, oncology and fertility—and I was thrilled to share my thoughts and passion for this new field and the goal of helping young women with cancer protect their future reproductive health.
Oncofertility was just an idea 10 years ago. Today it is a distinct field of medicine, offering new hope to cancer patients who will survive their disease and have fertility options that prior generations lacked. In the last year alone, 90 percent of young cancer patients at my institution received information about fertility as part of their cancer care. I cannot describe just how monumental this shift is for medical practice, with reproductive specialists and oncologists working together to improve patient care. For the patients at my institution, the discussions taking place between these two very different disciplines have led to interventions—banking eggs, sperm, embryos or tissue—with the goal of preserving the option to have a future family. For some of our cancer survivors, that future is now, and they are the proud parent of a child they thought they might not be able to have.
Bench to bedside to babies – oncofertility – is now part of the normal lexicon of centers of excellence around the globe as oncologists and reproductive specialists make fertility after cancer a priority at the time of diagnosis. Yet, when I delivered my remarks in Calgary less than a decade ago, I presented this kind of collaborative patient care as the future of medicine. So, what is my prediction for 2034?
Simply put, within the next 20 years, I expect to preside over the elimination of my field.
It sounds odd to create and then hope to eliminate an entire field of science and medicine, but that is my prediction and my hope. Let me tell you why. Even as science has created a medical arsenal of early diagnostics (genetic and blood-based), and more effective chemotherapeutics and radiation therapy approaches, I believe there is much more to come. Through science we will create new, smarter therapeutics that will treat the disease without causing collateral damage to the ovary and testes. We are learning more and more about the way the female egg and the male sperm respond to toxic drugs. Additionally, new technologies to better target drugs and radiation, as well as biologics that are specific to the disease, are on the horizon. Couple these technological advancements with a concerted effort to identify the triggers of cancer and to refocus our efforts on preventive measures that will either reduce the incidence of disease or diagnose it earlier and we will achieve better outcomes for patients. All of these advances – in cancer prevention, diagnosis, and treatment – will one day make it possible to address cancer without affecting future reproductive health, making the word oncofertility obsolete.
So, by 2034, I hope to once again become a regular reproductive scientist, continuing to work out the complexities of oocyte quality, ovarian follicle biology, and reproductive health; knowing that young women and men with cancer around the world need not worry about their ability to have a family one day; looking back fondly on the days when we needed oncofertility; and recognizing how continuing advances in cancer research allowed us to ultimately place oncofertility within the archives of medical history.
 Dr. Teresa Woodruff is the Thomas J. Watkins Professor of Obstetrics & Gynecology, the Vice Chair of Research (OB/GYN), the Chief of the Division of Reproductive Science in Medicine, Feinberg School of Medicine and Professor of Molecular Biosciences at the Weinberg College of Arts and Sciences at Northwestern University. She is also the Director of the Northwestern University Women’s Health Research Institute. Her work has been supported by the National Institutes of Health and the National Science Foundation.
Dr. Teresa Woodruff is the Thomas J. Watkins Professor of Obstetrics & Gynecology, the Vice Chair of Research (OB/GYN), the Chief of the Division of Reproductive Science in Medicine, Feinberg School of Medicine and Professor of Molecular Biosciences at the Weinberg College of Arts and Sciences at Northwestern University. She is also the Director of the Northwestern University Women’s Health Research Institute. Her work has been supported by the National Institutes of Health and the National Science Foundation.
 Today, Many women live beyond age 80 and as a result may be postmenopausal for over 30 years. Each woman has a unique range of symptoms. Hormone therapy has been widely prescribed since the early 60s despite limited research to relieve unpleasant menopausal symptoms. However, alarms were raised in the 1990's that have led to a whole battery of new research on hormone therapy that continues to this day.
Today, Many women live beyond age 80 and as a result may be postmenopausal for over 30 years. Each woman has a unique range of symptoms. Hormone therapy has been widely prescribed since the early 60s despite limited research to relieve unpleasant menopausal symptoms. However, alarms were raised in the 1990's that have led to a whole battery of new research on hormone therapy that continues to this day.
 This year, September 16 marked
This year, September 16 marked  Ads for ED drugs like Viagra and Cialis run across our TVs , newspapers and social media sites every day. Will there ever be a similar product for women? Here is an
Ads for ED drugs like Viagra and Cialis run across our TVs , newspapers and social media sites every day. Will there ever be a similar product for women? Here is an  Keeping skin healthy is important, especially as people get older, according to Bethanee J. Schlosser, MD, PhD, FAAD, assistant professor of dermatology and director of Women’s Skin Health at Northwestern University, Feinberg School of Medicine in Chicago. Dr. Schlosser is also on the Leadership Council of the Women's Health Research Institute. Read some helpful tips
Keeping skin healthy is important, especially as people get older, according to Bethanee J. Schlosser, MD, PhD, FAAD, assistant professor of dermatology and director of Women’s Skin Health at Northwestern University, Feinberg School of Medicine in Chicago. Dr. Schlosser is also on the Leadership Council of the Women's Health Research Institute. Read some helpful tips 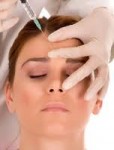 Minimally invasive cosmetic procedures, including fillers, neurotoxins and laser and energy device procedures are exceedingly safe and have essentially no risk of serious adverse events, reports a new Northwestern Medicine® study that analyzed more than 20,000 procedures around the country.
Minimally invasive cosmetic procedures, including fillers, neurotoxins and laser and energy device procedures are exceedingly safe and have essentially no risk of serious adverse events, reports a new Northwestern Medicine® study that analyzed more than 20,000 procedures around the country. Dr. Teresa Woodruff is the Thomas J. Watkins Professor of Obstetrics & Gynecology, the Vice Chair of Research (OB/GYN), the Chief of the Division of Reproductive Science in Medicine, Feinberg School of Medicine and Professor of Molecular Biosciences at the Weinberg College of Arts and Sciences at Northwestern University. She is also the Director of the Northwestern University Women’s Health Research Institute. Her work has been supported by the National Institutes of Health and the National Science Foundation.
Dr. Teresa Woodruff is the Thomas J. Watkins Professor of Obstetrics & Gynecology, the Vice Chair of Research (OB/GYN), the Chief of the Division of Reproductive Science in Medicine, Feinberg School of Medicine and Professor of Molecular Biosciences at the Weinberg College of Arts and Sciences at Northwestern University. She is also the Director of the Northwestern University Women’s Health Research Institute. Her work has been supported by the National Institutes of Health and the National Science Foundation. The recent announcement by the NIH that it would change its funding decisions to address the lack of female animals and cells in early bench research, was indeed good news. Yet, to date, no funding rules have changed. To be fair, the NIH did issue a request for information from the research community so they could better understand the barriers to inclusion. They are now reviewing those comments.
The recent announcement by the NIH that it would change its funding decisions to address the lack of female animals and cells in early bench research, was indeed good news. Yet, to date, no funding rules have changed. To be fair, the NIH did issue a request for information from the research community so they could better understand the barriers to inclusion. They are now reviewing those comments. The
The