 Women may soon bid farewell to birth control pills and welcome a new type of contraception in the form of microchip implants. An MIT startup backed by the Bill Gates Foundation plans to start pre-clinical testing for the birth control chip next year and pave the way for a possible market debut in 2018.
Women may soon bid farewell to birth control pills and welcome a new type of contraception in the form of microchip implants. An MIT startup backed by the Bill Gates Foundation plans to start pre-clinical testing for the birth control chip next year and pave the way for a possible market debut in 2018.The fingernail-size microchip implant holds enough 30-microgram daily doses of levonorgestrel—a hormone already used in several contraceptives—to last for 16 years. Women who received the implant under the skin of buttocks, upper arm or abdomen would also get a remote control that allows them to halt or restart the implant whenever they like, according to MIT Technology Review.
MicroCHIPS, the MIT startup behind the birth control implant, developed a clever design for a titanium and platinum seal that temporarily melts when an internal battery sends an electric charge running through the seal. That lasts just long enough for the melted seal to release the daily dose of levonorgestrel from the microchip reservoirs.
The microchip technology's latest mission first came about when Bill Gates visited the MIT lab of Robert Langer and challenged researchers to come up with a birth control method that women could control themselves and would also last for many years. Langer, an MIT professor who already holds 1,050 patents worldwide, thought of using the controlled release microchip technology that he and his colleagues had developed in the 1990s.
MicroCHIPS had previously demonstrated how the microchip technology could release daily doses of an osteoporosis drug during human clinical trials detailed in the 16 Feb 2012 online edition of the journal Science Translational Medicine. The new application for the microchips—each measuring 20 x 20 x 7 millimeters—could potentially revolutionize the level of control women have over their birth control technologies.
The biggest difference that the MicroCHIPS technology brings comes from giving women control over starting and stopping birth control regimens that can otherwise work for years without requiring regular attention. By comparison, existing contraceptive implants require a trip to the local clinic or hospital for removal if a woman wants to stop using the implant.
Any device offering wireless control for its users also runs the risk of being hacked. But Robert Farra, president and CEO of MicroCHIPS, told BBC News that their technology included secure encryption to prevent outsiders from blocking or reprogramming the implants wirelessly. As an added precaution, the remote control can only communicate with the microchip implant across a distance equivalent to skin contact.
Source: IEEE Spectrum By Jeremy Hsu

 Most people know that human clinical trials are critical to prove safety and efficacy in new medications. This is also true for medical devices yet a recent study indicated that only 14% of device studies included sex as a key outcome measure, and only 4% included a subgroup analysis for female participants. The differences in anatomy and physiology, as well as other factors in men and women, can lead to devices working less effectively and safely.
Most people know that human clinical trials are critical to prove safety and efficacy in new medications. This is also true for medical devices yet a recent study indicated that only 14% of device studies included sex as a key outcome measure, and only 4% included a subgroup analysis for female participants. The differences in anatomy and physiology, as well as other factors in men and women, can lead to devices working less effectively and safely. Women who had their last child at age 33 years or older were more likely to reach extremes of longevity, according to an analysis published online June 23 in Menopause.
Women who had their last child at age 33 years or older were more likely to reach extremes of longevity, according to an analysis published online June 23 in Menopause. The heart is more forgiving than you may think -- especially to adults who try to take charge of their health, a new Northwestern Medicine® study has found.
The heart is more forgiving than you may think -- especially to adults who try to take charge of their health, a new Northwestern Medicine® study has found. Yesterday, in a 5-4 decision, the Supreme Court ruled that “requiring family-owned corporations to pay for insurance coverage for contraception under the Affordable Care Act violated a federal law protecting religious freedom.” Justice Samuel A. Alito Jr. conceded that the government does have a “compelling interest in making sure women have access to contraception,” but that there are ways of providing that access without “violating the companies’ religious rights.”
Yesterday, in a 5-4 decision, the Supreme Court ruled that “requiring family-owned corporations to pay for insurance coverage for contraception under the Affordable Care Act violated a federal law protecting religious freedom.” Justice Samuel A. Alito Jr. conceded that the government does have a “compelling interest in making sure women have access to contraception,” but that there are ways of providing that access without “violating the companies’ religious rights.” The use of certain acne products containing the active ingredients benzoyl peroxide or salicylic acid can cause rare but serious and potentially life-threatening allergic reactions or severe irritation according to the Food and Drug Administration (FDA). The acne products the FDA is warning about are sold over-the-counter (OTC) and applied to the skin (topical). Their serious allergic reactions differ from the less harmful irritations already included in the product Drug Facts labels, which include burning, dryness, itching, peeling, redness, and slight swelling where the product is applied.
The use of certain acne products containing the active ingredients benzoyl peroxide or salicylic acid can cause rare but serious and potentially life-threatening allergic reactions or severe irritation according to the Food and Drug Administration (FDA). The acne products the FDA is warning about are sold over-the-counter (OTC) and applied to the skin (topical). Their serious allergic reactions differ from the less harmful irritations already included in the product Drug Facts labels, which include burning, dryness, itching, peeling, redness, and slight swelling where the product is applied. Disturbing reports about mismanagement at the Veteran's Affairs (VA) medical centers had led to Congressional action. One of our Leadership Council members at the Women's Health Research Institute, Melina R. Kibbe, MD, a vascular surgeon at the Feinberg School of Medicine and the Jesse Brown Veterans Medical Center in Chicago is a co author of a JAMA commentary that makes some well-thought out recommendations for change. While outrage is running rampant, it's time for everyone to concentrate on fixing the system that is critical to the men and women who defend our country. To view the commentary, visit
Disturbing reports about mismanagement at the Veteran's Affairs (VA) medical centers had led to Congressional action. One of our Leadership Council members at the Women's Health Research Institute, Melina R. Kibbe, MD, a vascular surgeon at the Feinberg School of Medicine and the Jesse Brown Veterans Medical Center in Chicago is a co author of a JAMA commentary that makes some well-thought out recommendations for change. While outrage is running rampant, it's time for everyone to concentrate on fixing the system that is critical to the men and women who defend our country. To view the commentary, visit 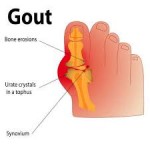 Research presented at the European Congress of Rheumatology indicated that “women have different predisposing risk factors for gout than do men, who more often fit the stereotypical profile of patients with gout who consume foods that increase the risk of the disease.” The research, “based on data collected from participants in the Consortium of Rheumatology Researchers of North America (CORRONA) gout registry,” indicated that “women with gout were more likely to have taken predisposing medications and to have more gout-associated comorbidities, whereas men were more likely to consume foods linked to the disorder, such as alcohol and red meat, according to Dr. Leslie Harrold, scientific director of the CORRONA gout registry.” This is just one more area that needs more exploration of sex differences! Source: Rheumatology News (6/25, Sullivan)
Research presented at the European Congress of Rheumatology indicated that “women have different predisposing risk factors for gout than do men, who more often fit the stereotypical profile of patients with gout who consume foods that increase the risk of the disease.” The research, “based on data collected from participants in the Consortium of Rheumatology Researchers of North America (CORRONA) gout registry,” indicated that “women with gout were more likely to have taken predisposing medications and to have more gout-associated comorbidities, whereas men were more likely to consume foods linked to the disorder, such as alcohol and red meat, according to Dr. Leslie Harrold, scientific director of the CORRONA gout registry.” This is just one more area that needs more exploration of sex differences! Source: Rheumatology News (6/25, Sullivan)  Delinquency in youth predicts a significantly higher rate of violent death in adulthood -- especially from firearms -- and females are among the most vulnerable, reports a new Northwestern Medicine® study.
Delinquency in youth predicts a significantly higher rate of violent death in adulthood -- especially from firearms -- and females are among the most vulnerable, reports a new Northwestern Medicine® study. Bone health in women has raised a lot of concern and generated many recommendations. Current guidelines from the U.S. Preventive Services Task Force (USPSTF) recommends that women ages 65 and older be screened routinely for osteoporosis. To reduce bone loss and decrease risk of fractures, calcium and vitamin D recommends have been outlined as well; premenopausal women should consume at least 1,000 mg per day and postmenopausal women should consume 1,200 mg per day (total diet and supplement). But are women taking too much calcium?
Bone health in women has raised a lot of concern and generated many recommendations. Current guidelines from the U.S. Preventive Services Task Force (USPSTF) recommends that women ages 65 and older be screened routinely for osteoporosis. To reduce bone loss and decrease risk of fractures, calcium and vitamin D recommends have been outlined as well; premenopausal women should consume at least 1,000 mg per day and postmenopausal women should consume 1,200 mg per day (total diet and supplement). But are women taking too much calcium?