 The U.S. Food and Drug Administration today approved a new genetic test that will help health care professionals determine if women with breast cancer are HER2-positive and, therefore, candidates for Herceptin (trastuzumab), a commonly used breast cancer treatment.
The U.S. Food and Drug Administration today approved a new genetic test that will help health care professionals determine if women with breast cancer are HER2-positive and, therefore, candidates for Herceptin (trastuzumab), a commonly used breast cancer treatment.
The test, called Inform Dual ISH, allows for measurement of the number of copies of the HER2 gene in tumor tissue. The HER2 gene is located on chromosome 17 in human cells. An excessive amount of the protein produced by the gene is found in some types of cancer cells, including breast cancer cells.
The Inform Dual ISH test allows lab personnel to count the number of copies of HER2 genes on chromosome 17 in a small sample of the breast tumor. The sample is stained with chemicals that cause copies of HER2 genes and chromosome 17 to change color. Copies of the HER2 gene appear black and copies of chromosome 17 appear red. These color changes can be seen under a standard microscope.
This feature allows lab personnel to see and count copies of chromosome 17 and HER2 genes on the same slide, similar to HER2 amplification measurements that have traditionally only been available using fluorescence microscopes. The Inform Dual ISH, however, allows lab staff to see the HER2 and chromosome 17 signals directly under a microscope, for longer periods of time.
The FDA based its approval of the Inform Dual ISH on a U.S. study involving tumor samples from 510 patients with breast cancer. This study showed that the test was effective in confirming that a patient’s tumor sample contained more than the normal number of copies of the HER2 gene in 96 percent of the HER2 positive tumor samples. Patients with more than the normal number of copies of the HER2 gene are considered candidates for Herceptin therapy. About 20 percent of women diagnosed with breast cancer are HER2-positive.

 Today, June 2, 2011, the U.S. Food and Drug Administration (FDA) warned women not to substitute breast thermography for mammography to screen for breast cancer. Some health care providers are promoting breast thermography on their websites and claiming that thermography is superior to mammography as a screening method for breast cancer, because it does not require radiation exposure or breast compression.
Today, June 2, 2011, the U.S. Food and Drug Administration (FDA) warned women not to substitute breast thermography for mammography to screen for breast cancer. Some health care providers are promoting breast thermography on their websites and claiming that thermography is superior to mammography as a screening method for breast cancer, because it does not require radiation exposure or breast compression. Choosing a treatment option for breast cancer can be almost as confusing and frightening as the diagnosis itself. But it doesn’t have to be. A new study from the University of Michigan has found that women make smarter choices about treatments when they receive information and make decisions in small doses rather than all at once.
Choosing a treatment option for breast cancer can be almost as confusing and frightening as the diagnosis itself. But it doesn’t have to be. A new study from the University of Michigan has found that women make smarter choices about treatments when they receive information and make decisions in small doses rather than all at once. The results of the first study to examine the relationship between menopausal symptoms and breast cancer risk are available online ahead of the February print issue of
The results of the first study to examine the relationship between menopausal symptoms and breast cancer risk are available online ahead of the February print issue of 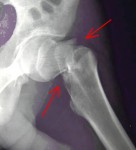 Painful Hip Fractures Strike Breast Cancer Survivors
Painful Hip Fractures Strike Breast Cancer Survivors The U.S. Food and Drug Administration (FDA) has approved the first 3-D mammography imaging system that may boost accuracy in breast cancer detection and diagnosis.
The U.S. Food and Drug Administration (FDA) has approved the first 3-D mammography imaging system that may boost accuracy in breast cancer detection and diagnosis. Starting Hormone Therapy at Menopause Increases Breast Cancer Risk
Starting Hormone Therapy at Menopause Increases Breast Cancer Risk After an intensive review of known cases of a rare form of cancer in breast implant recipients, the Food and Drug Administration says women with implants may have a very small, but increased risk of developing anaplastic large cell lymphoma, or ALCL.
After an intensive review of known cases of a rare form of cancer in breast implant recipients, the Food and Drug Administration says women with implants may have a very small, but increased risk of developing anaplastic large cell lymphoma, or ALCL. While the role of alcohol consumption has been established as a risk factor for breast cancer, most of the research has focused the relationship on hormonally sensitive breast cancers. A
While the role of alcohol consumption has been established as a risk factor for breast cancer, most of the research has focused the relationship on hormonally sensitive breast cancers. A  Menopause and the appropriate way to handle symptoms continues to be a lively topic of conversation. Much of this discussion is based on findings from the landmark
Menopause and the appropriate way to handle symptoms continues to be a lively topic of conversation. Much of this discussion is based on findings from the landmark