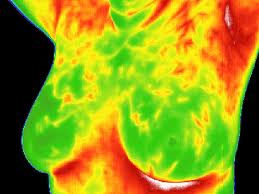
 Today, June 2, 2011, the U.S. Food and Drug Administration (FDA) warned women not to substitute breast thermography for mammography to screen for breast cancer. Some health care providers are promoting breast thermography on their websites and claiming that thermography is superior to mammography as a screening method for breast cancer, because it does not require radiation exposure or breast compression.
Today, June 2, 2011, the U.S. Food and Drug Administration (FDA) warned women not to substitute breast thermography for mammography to screen for breast cancer. Some health care providers are promoting breast thermography on their websites and claiming that thermography is superior to mammography as a screening method for breast cancer, because it does not require radiation exposure or breast compression.
The FDA is unaware of any valid scientific evidence showing that thermography, when used alone, is effective in screening for breast cancer. To date, the FDA has not approved a thermography device (also referred to as a telethermographic device) for use as a stand-alone to screen or diagnose breast cancer. However, the FDA has previously cleared thermography devices for use only as an adjunctive diagnostic tool for breast cancer screening and diagnosis. Therefore, thermography devices should not be used as a stand-alone method for breast cancer screening or diagnosis.
Additional information can be found at the LINK.

Comments