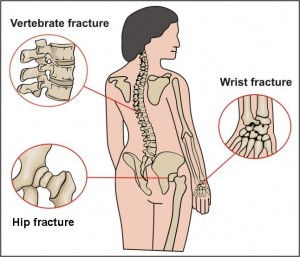
 A recent publication from the National Osteoporosis Foundation reported that many women with postmenopausal osteoporosis underestimate their risk for fractures. This is particularly alarming when it is coupled with new information released from the Food and Drug Administration (FDA) that found potential increased risk of fracture of the hip, wrist and spine if you take certain drugs for heartburn, acid reflux, or ulcers.
A recent publication from the National Osteoporosis Foundation reported that many women with postmenopausal osteoporosis underestimate their risk for fractures. This is particularly alarming when it is coupled with new information released from the Food and Drug Administration (FDA) that found potential increased risk of fracture of the hip, wrist and spine if you take certain drugs for heartburn, acid reflux, or ulcers.
The drugs under study belong to a class of medications called proton pump inhibitors (PPIs), which work by reducing the amount of acid in the stomach. They are available as prescriptions and as over-the-counter medications. These drugs treat conditions like gastroesophageal reflux disease (GERD), heartburn and ulcers of the stomach and small intestines.
Prescription PPIs include: Nexium, Dexilant, Prilosec, Zegerid, Prevacid, Protonix, Aciphex and Vimovo. The over-the-counter PPIs are: Prilosec OTC and Zegerid OTC (omeprazole), and Prevacid 24R (lansoprazole).
According to the FDA, consumers should:
- NOT stop taking your PPI unless told to by your health professionals. They are an effective treatment for many GI disorders.
- Be aware that an increased risk of fractures of the hip, wrist, and spine have been reported in some studies with the greatest increased risk for these fractures among those who receive high doses of these medications or use them for a year or longer.
- Read labels and follow instructions carefully and talk to your health provider if you have questions.
- Be aware that the over-the-counter PPIs should only be used as directed for 14 days for the treatment of frequent heartburn. If your heartburn persists, talk to your health professional. No more than three 14-day treatment courses should be used per year.
- Report any side effects from the use of PPIs to the FDA MedWatch Adverse Reporting Program.
To read the full FDA article click here. Osteoporosis affects 8 million women and 2 million men in the U.S.

 The FDA recently issued the following communication report: Patients and healthcare professionals may have questions about oral bisphosphonate medications and atypical subtrochanteric femur fractures – fractures in the bone just below the hip joint. Oral bisphosphonates are commonly prescribed to prevent or treat osteoporosis in postmenopausal women. Common brand names of medications in this class include Fosamax, Actonel, Boniva, and Reclast. Recent news reports have raised the question about whether there is an increased risk of this type of fracture in patients with osteoporosis using these medications. At this point, the data that FDA has reviewed have not shown a clear connection between bisphosphonate use and a risk of atypical subtrochanteric femur fractures. FDA is working closely with outside experts, including members of the recently convened American Society of Bone and Mineral Research Subtrochanteric Femoral Fracture Task Force, to gather additional information that may provide more insight into this issue.
The FDA recently issued the following communication report: Patients and healthcare professionals may have questions about oral bisphosphonate medications and atypical subtrochanteric femur fractures – fractures in the bone just below the hip joint. Oral bisphosphonates are commonly prescribed to prevent or treat osteoporosis in postmenopausal women. Common brand names of medications in this class include Fosamax, Actonel, Boniva, and Reclast. Recent news reports have raised the question about whether there is an increased risk of this type of fracture in patients with osteoporosis using these medications. At this point, the data that FDA has reviewed have not shown a clear connection between bisphosphonate use and a risk of atypical subtrochanteric femur fractures. FDA is working closely with outside experts, including members of the recently convened American Society of Bone and Mineral Research Subtrochanteric Femoral Fracture Task Force, to gather additional information that may provide more insight into this issue.