 Medicaid patients in Illinois (our home state!) could gain increased access to contraception under policy changes proposed August 18 by the Department of Healthcare and Family Services, according to the Chicago Tribune. Health care providers would receive more money for providing vasectomies to men and birth control to women under the proposal, which also includes a possible new referral requirement for Roman Catholic providers and others that object to contraception.
Medicaid patients in Illinois (our home state!) could gain increased access to contraception under policy changes proposed August 18 by the Department of Healthcare and Family Services, according to the Chicago Tribune. Health care providers would receive more money for providing vasectomies to men and birth control to women under the proposal, which also includes a possible new referral requirement for Roman Catholic providers and others that object to contraception.
The department expects to implement most of the proposed changes this fall, but department Director Julie Hamos said Medicaid will immediately start paying more toward the cost of long-term contraception at walk-in providers such as Planned Parenthood clinics.
Hamos said her department proposed the changes in part to address the recent Supreme Court decision that allowed some companies to exclude contraceptives from their employees' insurance coverage on religious grounds. Oklahoma-based arts-and-crafts retailer Hobby Lobby, owned by evangelical Christians, sued over a requirement under the Affordable Care Act to cover contraceptives.
The court's decision was of "extreme concern" to Gov. Pat Quinn and state health officials, Hamos said. The new proposal affects residents covered under Medicaid, not by employers, but Hamos said the court's decision brought new focus to the issue, spurring the department to announce the proposal quickly.
"It is an opportune time when women across the country are paying attention … that's a time that we can really use that attention to focus on what's available to them through Medicaid," Hamos said. She noted that the change could help low-income women who shift between Medicaid and employer coverage as their employment situations change. Unplanned pregnancies constitute a major cost among the approximately 1 million women of childbearing age enrolled in Medicaid in Illinois, Hamos said. About 3 million Illinoisans in all are enrolled, and the number is set to expand under the Affordable Care Act, commonly known as Obamacare.
Expanded family planning has succeeded at saving money in other states, Hamos said, citing a Colorado initiative that she said cut teen birthrates by 40 percent from 2009 through 2013, reduced abortions and saved the state $42.5 million in 2010.
The Illinois proposal aims to coax more health care providers into expanding family planning services by increasing their reimbursement rates starting Oct. 1. Payments for vasectomies and intrauterine devices would be doubled. Hamos said the department is working with companies that manufacture IUDs to ensure they're on health care providers' shelves when needed.
This Chicago Tribune story was produced in partnership with Kaiser Health News, an editorially independent program of the Kaiser Family Foundation.

 Women may soon bid farewell to birth control pills and welcome a new type of contraception in the form of microchip implants. An MIT startup backed by the Bill Gates Foundation plans to start pre-clinical testing for the birth control chip next year and pave the way for a possible market debut in 2018.
Women may soon bid farewell to birth control pills and welcome a new type of contraception in the form of microchip implants. An MIT startup backed by the Bill Gates Foundation plans to start pre-clinical testing for the birth control chip next year and pave the way for a possible market debut in 2018. Yesterday, in a 5-4 decision, the Supreme Court ruled that “requiring family-owned corporations to pay for insurance coverage for contraception under the Affordable Care Act violated a federal law protecting religious freedom.” Justice Samuel A. Alito Jr. conceded that the government does have a “compelling interest in making sure women have access to contraception,” but that there are ways of providing that access without “violating the companies’ religious rights.”
Yesterday, in a 5-4 decision, the Supreme Court ruled that “requiring family-owned corporations to pay for insurance coverage for contraception under the Affordable Care Act violated a federal law protecting religious freedom.” Justice Samuel A. Alito Jr. conceded that the government does have a “compelling interest in making sure women have access to contraception,” but that there are ways of providing that access without “violating the companies’ religious rights.”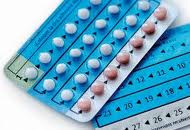 [09-26-2011] The U.S. Food and Drug Administration (FDA) is informing the public that it has not yet reached a conclusion, but remains concerned, about the potential increased risk of blood clots with the use of drospirenone-containing birth control pills. FDA has completed its review of the two 2011 studies that evaluated the risk of blood clots for women who use drospirenone-containing birth control pills, previously mentioned in FDA's Drug Safety Communication issued on May 31, 2011. FDA is continuing its review of a separate FDA-funded study that evaluated the risk of blood clots in users of several different hormonal birth control products (contraceptives). Preliminary results of the FDA-funded study suggest an approximately 1.5-fold increase in the risk of blood clots for women who use drospirenone-containing birth control pills compared to users of other hormonal contraceptives.
[09-26-2011] The U.S. Food and Drug Administration (FDA) is informing the public that it has not yet reached a conclusion, but remains concerned, about the potential increased risk of blood clots with the use of drospirenone-containing birth control pills. FDA has completed its review of the two 2011 studies that evaluated the risk of blood clots for women who use drospirenone-containing birth control pills, previously mentioned in FDA's Drug Safety Communication issued on May 31, 2011. FDA is continuing its review of a separate FDA-funded study that evaluated the risk of blood clots in users of several different hormonal birth control products (contraceptives). Preliminary results of the FDA-funded study suggest an approximately 1.5-fold increase in the risk of blood clots for women who use drospirenone-containing birth control pills compared to users of other hormonal contraceptives.