 Today, the scientific journal, Nature, released a Commentary by Institute Director Teresa Woodruff and two of her post doctorate students that we hope will open up the dialogue about the current status of women's health research. For the reader's convenience, the entire press release is listed below as well as a link to the article. More examples of sex bias will be featured on this blog over the next two weeks, so we hope you will tag this site!
Today, the scientific journal, Nature, released a Commentary by Institute Director Teresa Woodruff and two of her post doctorate students that we hope will open up the dialogue about the current status of women's health research. For the reader's convenience, the entire press release is listed below as well as a link to the article. More examples of sex bias will be featured on this blog over the next two weeks, so we hope you will tag this site!
CHICAGO --- Women remain vastly underrepresented in biomedical research despite significant differences in the way they experience many diseases, medications and therapies compared to men. Despite federal mandates to include women in studies, there is much that still needs to be done, says Teresa Woodruff, a leading women’s health scientist at Northwestern University Feinberg School of Medicine, in a June 9 commentary in the journal Nature.
“It’s time for the sex bias in basic research and clinical medicine to end,” writes senior author Woodruff, executive director of the Institute for Women's Health Research and the Thomas J. Watkins Professor of Obstetrics and Gynecology at Feinberg. This bias, she says, has an enormous effect on women’s health, resulting, for example, in delayed diagnosis of cardiovascular disease, the leading killer of women, and in adverse reactions to medication. Alison Kim and Candace Tingen, post-doctoral fellows at Feinberg, are coauthors on the paper.
Women need to be adequately represented in studies, and results need to be specifically designed and analyzed to determine sex differences, the authors note, in order for both men and women to receive more tailored care. Understanding sex as a determinant of disease and care is the first step towards personalized care for every patient. Sex differences in the incidence, prevalence, symptoms and severity of disease have already been shown in autoimmune diseases such as rheumatoid arthritis and multiple sclerosis; in psychological disorders including depression, autism, eating disorders and schizophrenia; and in asthma and several types of cancer.
“Differences are particularly acute in cardiovascular disease, the leading cause of death for both men and women,” Woodruff writes. Women in the early stages of cardiovascular disease may experience fatigue, abdominal discomfort and back, jaw or neck pain, all of which are considered atypical because diagnostics were mainly established from research conducted primarily on men. “As a result, women can be subject to potentially life-threatening delays before crucial diagnostic tests are administered,” she said. And some of those tests, like exercise electrocardiography, can’t detect cardiovascular disease as well in women as in men.
Northwestern is a good example of making significant strides in treating women with heart disease and in sex-balanced health research, Woodruff noted. She pointed to Feinberg’s Institute for Women’s Health Research, which supports clinical research trials that focus on women’s health and sex-balanced research, and the institute’s Illinois Women’s Health Registry, a growing database of 4,500 potential study subjects, that has encouraged Northwestern researchers to do more research in sex differences. About 1,000 of these women have participated in trials.
In addition, the Center for Women’s Cardiovascular Health at the Bluhm Cardiovascular Institute of Northwestern Memorial Hospital has a unique clinical program focusing on women’s heart health. Women also respond differently to medication than men, yet drugs are rarely prescribed accordingly, according the Nature article. “This may be part of the reason why women are 1.5 times more likely to develop an adverse reaction to prescription drugs than men,” Woodruff notes. For example, a 2005 study of new drug applications found that even the drugs that had substantial differences in how they were metabolized by women and men did not have sex-specific dosage recommendations on the labels.
How to fix the void? Among the authors’ suggestions:
- Journals should indicate if results are in male or female animal models.
- Regulatory and funding agencies should require appropriate representation of both sexes in human and animal trials and require researchers to consider sex differences when they analyze data.
- Educate doctors in the clinical importance of sex differences. The Food and Drug Administration also should mandate that medication dosages be sex-specific based on clinical studies.
To read the complete article, click here.
NORTHWESTERN NEWS: www.northwestern.edu/newscenter/


 concerning, it was just early menopause. Of course, any atypical health symptoms need to be checked out, no matter how old you are!
concerning, it was just early menopause. Of course, any atypical health symptoms need to be checked out, no matter how old you are! An article was released in the June 10, 2010 edition of the well-respected journal, Nature, that raises the question of doing research studies on pregnant women. Women get colds, the flu, infections and other diseases during their pregnancies that have nothing to do with their mom-to-be status. Many women simply "toughen it out" hoping their condition is just a virus that will run its course. Other times, they rely on their obstetrician's experience with other patients who may have been prescribed an antedote that the physician has used successfully in her/his practice, but has not actually been studied in well-designed research studies that included pregnant women.
An article was released in the June 10, 2010 edition of the well-respected journal, Nature, that raises the question of doing research studies on pregnant women. Women get colds, the flu, infections and other diseases during their pregnancies that have nothing to do with their mom-to-be status. Many women simply "toughen it out" hoping their condition is just a virus that will run its course. Other times, they rely on their obstetrician's experience with other patients who may have been prescribed an antedote that the physician has used successfully in her/his practice, but has not actually been studied in well-designed research studies that included pregnant women.
 According to the World Health Organization, alcohol is one of the most significant risk factors for diseases including chronic conditions like cancer, diabetes, and heart disease. Compared with men, women become more cognitively impaired by alcohol and are more susceptible to alcohol-related organ damage. Women develop damage with less intake and over a shorter period of time than men. When men and women of the same weight consume equal amounts of alcohol, women have higher blood alcohol concentrations. This is due in part because women have proportionately more body fat and a lower volume of body water compared with men of similar weight. This leads to women having a higher concentration of alcohol because there is less volume of water to dilute the alcohol.
According to the World Health Organization, alcohol is one of the most significant risk factors for diseases including chronic conditions like cancer, diabetes, and heart disease. Compared with men, women become more cognitively impaired by alcohol and are more susceptible to alcohol-related organ damage. Women develop damage with less intake and over a shorter period of time than men. When men and women of the same weight consume equal amounts of alcohol, women have higher blood alcohol concentrations. This is due in part because women have proportionately more body fat and a lower volume of body water compared with men of similar weight. This leads to women having a higher concentration of alcohol because there is less volume of water to dilute the alcohol. Today, the scientific journal, Nature, released a Commentary by Institute Director Teresa Woodruff and two of her post doctorate students that we hope will open up the dialogue about the current status of women's health research. For the reader's convenience, the entire press release is listed below as well as a link to the article. More examples of sex bias will be featured on this blog over the next two weeks, so we hope you will tag this site!
Today, the scientific journal, Nature, released a Commentary by Institute Director Teresa Woodruff and two of her post doctorate students that we hope will open up the dialogue about the current status of women's health research. For the reader's convenience, the entire press release is listed below as well as a link to the article. More examples of sex bias will be featured on this blog over the next two weeks, so we hope you will tag this site!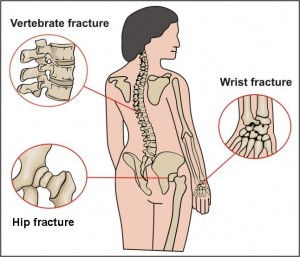 A recent publication from the National Osteoporosis Foundation reported that many women with postmenopausal osteoporosis underestimate their risk for fractures. This is particularly alarming when it is coupled with new information released from the Food and Drug Administration (FDA) that found potential increased risk of fracture of the hip, wrist and spine if you take certain drugs for heartburn, acid reflux, or ulcers.
A recent publication from the National Osteoporosis Foundation reported that many women with postmenopausal osteoporosis underestimate their risk for fractures. This is particularly alarming when it is coupled with new information released from the Food and Drug Administration (FDA) that found potential increased risk of fracture of the hip, wrist and spine if you take certain drugs for heartburn, acid reflux, or ulcers. according to researchers at the Johns Hopkins Bloomberg School of Public Health. They examined published data from numerous adult and child vaccine trials and found that sex is a fundamental, but often overlooked predictor of vaccine response that could help predict the efficacy of combating infectious disease. The review is featured in the May 2010 issue of The Lancet Infectious Diseases.
according to researchers at the Johns Hopkins Bloomberg School of Public Health. They examined published data from numerous adult and child vaccine trials and found that sex is a fundamental, but often overlooked predictor of vaccine response that could help predict the efficacy of combating infectious disease. The review is featured in the May 2010 issue of The Lancet Infectious Diseases. May 31st is World No Tobacco Day—an annual awareness day sponsored by the World Health Organization (WHO) since 1987 to draw worldwide attention to the tobacco epidemic and the preventable death and disease it causes. The theme for this year's World No Tobacco Day is "gender and tobacco, with an emphasis on marketing to women." Tobacco use is the leading cause of preventable death worldwide and is estimated to kill more than 5 million people each year.
May 31st is World No Tobacco Day—an annual awareness day sponsored by the World Health Organization (WHO) since 1987 to draw worldwide attention to the tobacco epidemic and the preventable death and disease it causes. The theme for this year's World No Tobacco Day is "gender and tobacco, with an emphasis on marketing to women." Tobacco use is the leading cause of preventable death worldwide and is estimated to kill more than 5 million people each year.