 The Women's Health Research Institute wrote the following letter advocating for better parameters for sex-inclusion at the basic science level:
The Women's Health Research Institute wrote the following letter advocating for better parameters for sex-inclusion at the basic science level:
On October 9, 2015, NIH released the final policy language associated with the inclusion of sex as a variable in scientific research together with additional guidance on improving the reproducibility of research findings (NOT-OD-16-011). The Women’s Health Research Institute has been a strong advocate for sex-inclusion and reporting and we are delighted that sex will now be part of scientific research in the same way time, temperature, dose and age are currently regarded. The primary literature documenting the need and value of these new policies is provided, as are a few FAQs that help guide the rationale behind this change. The new policy is effective for all grants submitted on/after Jan 25, 2016 and directions have been provided to CSR regarding study section review of these elements. We know that a change in policy requires added thought in the development of compliant applications and below we provide a guide and examples to enable your success. Please let us know if you have additional questions or suggestions for enabling fundamental and translational science at Northwestern University to succeed (womenshealthresearch@northwestern.edu).
Analyzing Sex in Preclinical Basic and Translational Research: FAQs
1. What is the new NIH policy and when will it be enforced?
NIH notified federally funded investigators that sex as a basic biological variable must be accounted for in NIH-funded preclinical research in May 2014. Since that time there have been a number of notices that provide additional information to prepare the community for this change (NOT-OD-16-012; NOT-OD-16-005; NOT-OD-16-004; NOT-OD-15-103; NOT-OD-15-102 ). On Nov 23, 2015, the NIH published guidance on where elements associated with this new plan must be included (NOT-OD-16-011). Specifically, investigators must include statements within the Approach section that provide “adequate plans to address relevant biological variables, such as sex, for studies in vertebrate animals or human subjects?’ You will not be required to test both sexes per se, but must justify why a single sex study is warranted. Language that provides guidance is provided at the end of this document. Additionally, the Women’s Health Research Institute staff is willing to assist by reviewing grant materials and will provide a letter that can be submitted with NU grant applications that indicate our investigators are aware of the notice and that we have a robust environment in which sex-inclusive studies are conducted. We will also support well-justified single sex studies in our letter of support. All grants submitted after Jan 25, 2016 will be required to include this language and CSR will be enforcing this section as a reviewable element.
The specific instructions from NIH are provided here (http://grants.nih.gov/reproducibility/faqs.htm#4760):
2. Is consideration of sex as a biological variable required for all grant applications?
NIH expects that sex as a biological variable will be factored into research designs, analyses, and reporting in vertebrate animal and human studies. NOT-OD-15-103 further communicates that a strong justification must be provided for applications proposing to study only one sex. NOT-OD-16-011 describes implementing rigor and transparency in NIH and AHRQ research grant applications and NOT-OD-16-012 describes implementation for career development grant applications. For more details on requirements and policy changes to NIH application forms for 2016 grant applications, please view guide notice NOT-OD-16-004. FAQs on these forms updates, including questions specifically addressing Vertebrate Animal subjects, can be found on the OER FAQs Form Updates 2016 page.
3. Which other "relevant biological variables" do applicants need to cover?
The biological variables that are relevant to the experimental design of the study, and the choice of animal model or human population to be included will vary with the scientific topic of the proposed research. Applicants are now directed to provide a justification for the species that are appropriate for the proposed research in the vertebrate animals section. The rationale for the number of subjects planned for study should now be explained in the approach section of the application.
NOT-OD-15-103 further communicates that a strong justification must be provided for applications proposing to study only one sex.
4. How should applicants address scientific rigor and in particular biological variables such as sex, when the research involves scarce animal resources?
Applicants are now directed to provide a justification that the species are appropriate for the proposed research in the vertebrate animals section. The rationale for the number of subjects planned for study should now be explained in the approach section of the application.
Applicants must provide strong justification to study only one sex. Such justification may include the study of sex-specific conditions or phenomena (e.g., ovarian or prostate cancer), or investigations in which the study of one sex is scientifically appropriate. The absence of evidence regarding sex differences in an area of research does not constitute strong justification to study only one sex. Cost also is not a consideration in determining whether both sexes are to be included in experiments.
5. Should sex as a biological variable be considered by IACUCs during review of animal use protocols or is this review the purview of NIH study sections?
Justification of the choice of sex(es) proposed in animal study protocols is not required by the PHS Policy on Humane Care and Use of Laboratory Animals, the Animal Welfare Act Regulations, or the Guide for the Care and Use of Laboratory Animals. Many IACUCs require identification of the sex of animals for facility management purposes, and some IACUCs ask for justification for studies proposing one sex. As stated in NOT-OD-15-102, accounting for sex as a biological variable should begin with the development of the research question and study design and will become part of review criteria for Approach during peer review.
6. May IACUCs approve animal use protocols that require a larger number of animals because of the new NIH requirement to include both sexes in research designs where relevant?
The PHS Policy on Humane Care and Use of Laboratory Animals, the Animal Welfare Act Regulations, and the Guide for the Care and Use of Laboratory Animals require animal use protocols to include a rationale for the number of animals to be used and require that the number proposed be limited to the minimum necessary to obtain valid results. It is the IACUC’s responsibility to review and confirm that a sound, objective, and logical reason has been provided for the number of animals proposed. If a PI has appropriately considered sex as a biological variable relevant to the study design, this is consistent with the federal requirement to use the minimum number, and acceptable to the IACUC.
Additional Information you may find useful. This material and other tools can be found on the Women’s Health Research Institute website (http://www.womenshealth.northwestern.edu). A series of references are provided below this section that can be used in your approach section.
7. What is sex?
“Sex” is biological and should not be used interchangeably with “gender.” Sex is a constellation of biological attributes that derive from sex chromosomes, reproductive organs, or specific hormones. There may be considerable overlap in “female” and “male” phenotypes, especially for secondary sex characteristics. For example here are overt differences in the weight of male and female animals at all ages. Importantly, every cell has a sex. While it is not necessary to directly study sex as a variable in all experiments it is important to know the sex chromosome status and be aware that differences between two cell lineages (for example iPS cells) may be predicated on their genetic organization.
8. What is the value of including both sexes in basic and translational research?
Designing studies that include the biological variability of sex early in the research pipeline promotes discovery and has the potential to save lives and money. For example, eight of ten drugs withdrawn from the U.S. market from 1997-2000 posed greater health risks for women than for men (1). Many such failures may be traced to inattention to sex as a variable in pre-clinical and clinical research (2-5). Examples of sex-inclusive breakthroughs at the bench and in translational research are found in a series of blog posts from the WHRI (See http://www.womenshealth.northwestern.edu/blog).
9. How many subjects do I need?
Most researchers are familiar with the use of statistical power analyses to estimate the number of subjects needed to detect an effect relative to a control.
- To demonstrate an effect in both sexes (or any two groups), one must power the study for each separate group based on the estimated variance within each group. The total number is based on the sum of the required number for each individual group.
- To determine a sex difference (or a difference between any two groups), a sufficiently large sample size is needed to provide reliability in the detection of mean differences.
10. What if I don’t find a sex difference?
One cannot prove a negative, i.e. demonstrate absolutely NO sex difference, but confidence comes from effect size (Cohen’s d). For instance, if males and females differ significantly with respect to a given endpoint, but the effect size is very small (<0.2), this difference may not be worth pursuing. Conversely, if males and females differ only slightly on a specific outcome, but the effect size is large (>0.5), indicating a high degree of reliability (i.e., low variance), it is important to consider sex as a contingent variable. Not finding a sex difference is as important as finding one. However, clear evidence of this requires avoidance of accepting a false negative (Type I error) and not concluding a false positive (Type II error). Northwestern University Biostatistics Core can assist with sample sign assessment to meet the needs of investigators with all study design, including the inclusion of adequate numbers of males and females to determine sex differences.
11. If the effect size is small, can I return to studying one sex?
This would not be consistent with the intent of the new NIH policy, which is the inclusion of both sexes in preclinical research. If you have concluded there is not an important difference between male and female subjects, then you should continue to include both in all of your experiments. Moreover, even if the effect is the same in two groups, underlying differences of male and female biology suggests that different mechanisms may be at play and consistently excluding one sex may miss important discoveries.
12. Do I need to consider the estrous cycle?
The long held assumption that females are intrinsically more variable than males (because of the estrous cycle) has been discredited. In a recent meta-analysis that compared male and female mice without regard to estrous cycle stage, variability was not significantly greater in females than males for any of hundreds of endpoints (6). For most traits it is unnecessary to stage the estrous cycle in initial experiments. However, where there is evidence that reproductive hormones affect specific traits in humans or animal models, one needs to consider the reproductive phase of females in study design. Methods to cycle female animals can be found at: http://www.womenshealth.northwestern.edu/animal-research-methods. Members of the WHRI staff are also willing to show investigators how to maintain colonies of cycling female mice. Investigators should be aware that male hormones also cycle on a daily basis (7-8). Thus, standardizing the time of day that studies are done will improve overall reproducibility of data.
13. Will analyzing sex as a variable cost more money?
It depends on what you want to know. It will cost no more to report the sex of subjects, animals or cells. While this seems obvious, sex is rarely reported in the scientific and medical literature (9-10). It also will cost no more to adopt a strategy of 50/50 female and male animals and cells instead of 100% of one sex (except in rigorously defined cases). An analysis for sex should be done in the majority of experiments. Many traits may not differ between the sexes, suggesting that data from males and females can be combined without increasing overall sample size or total cost. Where a substantial sex difference is detected, sample size may have to be increased to generate sufficient power for statistical analysis; this is justified to ensure that findings apply to both sexes. Recognizing the issue of cost, the NIH in 2014 distributed $10.1 million to support sex inclusive research; it is anticipated that additional support will be provided going forward and we will continue to advocate that new funds will be a substantial catalyst to sex inclusive studies.
14. Why should I consider the sex of cells?
The sex of primary cells may explain significant variability in their responses to experimental perturbation and clinical potential. For example, skeletal muscle stem cells from females regenerate new tissue faster than stem cells from males (11-12). Similarly, bone marrow progenitor cells engrafted from females regenerate heart tissue faster than progenitor cells from males (13). Cells isolated from females also respond differently to stimulation and cellular stress than cells from males. Care may be needed to evaluate endpoints in steroid-free conditions as both phenol red and fetal bovine serum contain estrogenic compounds that may also affect cellular responses to stimulation (14).
15. What are rigorously defined exceptions?
Clearly, studies of prostate or uterine cancer will be conducted in only one sex. Similarly, studies of breast cancer in animal models probably do not require equal numbers of male and female subjects as breast cancer occurs predominantly in females, but can occur in a small percentage of males. However, one of the reasons for studying sex differences is the ability to discover what may underlie sex-specificity. For example, Dr. Melissa Brown ‘accidentally’ used male animals in a multiple sclerosis project study, in which the disease disproportionately impacts females (15). In so doing, our colleague discovered a new mechanism for by which the disease becomes more aggressive. Thus, sex inclusion may provide more information than could be uncovered in a single sex.
In the case of cell lines, many immortalized cell lines have become chromosomally unstable so that it is impossible to determine their original sex, and other chromosomal changes may be far more impactful than alterations in X or Y sequence or copy number. Thus, these lines cannot fairly be said to have a ‘sex’. Primary cell lines definitively have a ‘sex’ that may influence biological outcomes and should be examined.
16. Many variables affect our results, why is sex so important?
While many variables can influence an outcome, sex is not “just” another confounding variable, because no other variable affects a greater percentage of the population, nor is more evolutionarily fundamental. Furthermore, an enormous body of evidence documents the importance of the sex/gender variable for all biology, despite the fact that the issue has been historically massively understudied (1-24). Sex constitutes “low hanging fruit” in that, as an independent variable, it is easy to identify, almost completely binary, and with equal frequency of each “allele.” One can clearly see that sex, arguably more than any other variable, requires greater attention. For preclinical studies of drugs, devices, and biologics, sex represents a fundamental distinction in the quest toward personalized medicine. Perhaps most importantly, science takes into consideration many variables, and reports on them – time, temperature, dose for example. Sex is a variable that is at least equal to these determinants yet is often not even reported in the biological literature (16-18). The influence of sex on outcomes is critical to fundamental discovery and to the eventual utility of these discoveries to human health and is something the public finds hard to believe that we do not take this into account (19-20).
17. Who will be responsible for tracking the inclusion mandate?
Old PHS rules do not explicitly require a mechanism to track animal usage by investigators, it does require that proposals specify a rational for the approximate number of animals to be use and limits the project to that number. This may change with the new regulations and investigators should begin their own tracking mechanisms.
18. The new inclusion rules only affect federal funds; will researchers seek more private funding from PHARMA to expedite the process to new drugs and by pass the rules?
Pharma may be excluded from NIH policies but industry must meet FDA guidelines and the FDA is currently developing new requirements for drug and Device approval that will necessitate data on both sexes. Researchers will likely be required to show they tested their drugs and devices in both sexes.
19. What is gender? Does it apply to animals?
“Gender” is a constellation of socio-cultural processes that interact with, and thus influence, biology. In humans, gender refers to cultural attitudes that shape “feminine” and “masculine” behaviors that are learned and vary by culture, historical era, ethnicity, etc (21). In animal research, gender might be considered, for example, where different “standard” environmental housing conditions are based on sex differences in animal behaviors (22). Gender relations can also apply to social interactions between female and male animals, and between men or women researchers with male versus female animals (23). Environmental factors can interact with sex to produce different outcomes. In animal model (e.g., rats and mice) studies, care and management conditions can impact many traits (24). Examples include: 1) caging (single animals, mating pairs or single-sex groups), 2) source of animals (bred by commercial vendor and transported to research site vs. bred and reared at research site), 3) hygiene status (conventional including low level pathogens, specific pathogen-free, gnotobiotic, or germ-free, treatment with antibiotics), and 4) light cycle (including the time in the 24 hour cycle when samples are obtained). No study can address all of these environmental variables, but they should be equal and well controlled for male and female study subjects and reported.
20. What might an example of a compliant statement look like?
Non-compliant: Studies on male mice have formed the basis of our studies and we will therefore continue to use male animals as the sole sex in future studies.
Compliant: Studies on drug X will include equal numbers of male and female mice. There is no indication that a sex difference exists for this endpoint, therefore, we will use equal numbers of males and females with age ranges between 45-100 days.
Non-compliant: Immunologic disease predominantly affects females therefore we will study only females.
Compliant: Immunologic disease predominantly affects females and studies 1 and 2 will use females only to test how estrogen regulates signaling pathway Y. In experiment 3, we will use both males and females to examine whether male rats respond in the same way as the female rats with respect to signaling pathway Y. If so, we will move forward with mixed sex studies. If the females respond different to the hormone intervention we will report that males do not respond in the same way as females.
Non-compliant cell-based response: All mechanistic studies in Aims 2 and 3 will be performed with normal adult KCs in passage 2-3, given that most type 2 diabetic patients are adults.
Compliant cell-based response: All mechanistic studies in Aims 2 and 3 will be performed with normal adult KCs in passage 2-3, given that most type 2 diabetic patients are adults. We recognize the greater difficulty (i.e., requirement for viral gene transduction, slower growth) and cost of using KCs vs. immortalized KCs, but the many differences in molecular expression, signaling, and behavior between human primary vs. immortalized KCs make studying primary KCs more meaningful. In Aim 2, we will use single (not pooled) cells and compare the responses in male and female KCs (obtained from our SDRC cell banks) to determine sex-related differences.
Compliant cell-based response: All mechanistic studies in Aims 2 and 3 will be performed with normal adult KCs in passage 2-3, given that most type 2 diabetic patients are adults. All KCs cells will be pooled from both male and females adults.
Non-compliant: The studies in Aim 3 will be conducted on 12-14 week old male rats. No female rats will be used for these studies.
Compliant: The studies in Aim 3 will be conducted on 12-14 week old male rats. Our lab previously determined that therapy Z was equally effective in male and female rats (unpublished data); thus, further studies will be limited to a single sex
Compliant: The studies in Aim 3 will be conducted on 12-14 week old male rats. If therapy Z proves efficacious, we will extend our experiments to female rats of a similar age.
Compliant: The studies in Aim 3 will be conducted on 12-14 week old male and female rats.
References:
1. United States General Accounting Office. (2001). Drug Safety: Most Drugs withdrawn in Recent Years had Greater Health Risks for Women. Washington, DC: Government Publishing Office.
2. Collins, F. S., & Tabak, L. A. (2014). Policy: NIH plans to enhance reproducibility. Nature, 505(7485), 612-613.
3. Tingen, C. M., Kim, A. M., Wu, P. H., & Woodruff, T. K. (2010). Sex and sensitivity: the continued need for sex-based biomedical research and implementation. Womens Health (Lond Engl), 6(4), 511-516. doi: 10.2217/whe.10.45
4. Kim, A. M., Tingen, C. M., & Woodruff, T. K. (2010). Sex bias in trials and treatment must end. Nature, 465(7299), 688-689. doi: 10.1038/465688a
5. Woodruff, T. K. (2014). Sex, equity, and science. Proc Natl Acad Sci U S A, 111(14), 5063-5064. doi: 10.1073/pnas.1404203111
6. Prendergast, B. J., Onishi, K. G. & Zucker, I. (2014). Female mice liberated for inclusion in neuroscience and biomedical research. Neuroscience and Biobehavioral Reviews, 40, 1–5.
7. Dauchy, R. T., Wren, M. A., Dauchy, E. M., Hoffman, A. E., Hanifin, J. P., Warfield, B., . . . Blask, D. E. (2015). The influence of red light exposure at night on circadian metabolism and physiology in Sprague-Dawley rats. J Am Assoc Lab Anim Sci, 54(1), 40-50.
8. Chacon, F., Cano, P., Jimenez, V., Cardinali, D. P., Marcos, A., & Esquifino, A. I. (2004). 24-hour changes in circulating prolactin, follicle-stimulating hormone, luteinizing hormone, and testosterone in young male rats subjected to calorie restriction. Chronobiol Int, 21(3), 393-404.
9. Kong, B. Y., Haugh, I. M., Schlosser, B. J., Getsios, S., & Paller, A. S. (2015). Mind the Gap: Sex Bias in Basic Skin Research. J Invest Dermatol. doi: 10.1038/jid.2015.298
10. Yoon, D. Y., Mansukhani, N. A., Stubbs, V. C., Helenowski, I. B., Woodruff, T. K., & Kibbe, M. R. (2014). Sex bias exists in basic science and translational surgical research. Surgery, 156(3), 508-516. doi: 10.1016/j.surg.2014.07.001
11. Shah K., McCormack C., Bradbury N. (2014). Do You Know the Sex of Your Cells? American Journal of Physiology, Cell Physiology, 306 (1), C3-18.
12. Deasy, B., Lu, A., Tebbets, J., Feduska, J., Schugar, R., Pollett, J., Sun, B., Urish, K., Gharaibeh, B., Cao, B., Rubin, R., & Huard, J. (2007). A Role for Cell Sex in Stem Cell-Mediated Skeletal Muscle Regeneration: Female Cells Have Higher Muscle Regeneration Efficiency. The Journal of Cell Biology, 177 (1), 73-86.
13. Nelson, W.D., Zenovich, A. G., Ott, H. C., Stolen, C., Caron, G. J., Panoskaltsis-Mortari, A., Barnes, S.A. III, Xin, X., & Taylor, D. A. (2007). Sex-Dependent Attenuation of Plaque Growth After Treatment with Bone Marro Mononuclear Cells. Circulation Research, 101, 1319-27.
14. Paharkova-Vatchkova, V., Maldonado, R., Kovats ,S. (2004). Estrogen Preferentially Promotes the Differentiation of CD11c+ CD11b(Intermediate) Dendritic Cells from Bone Marrow Precursors. Journal of Immunology, 172(3),1426-36.
15. Russi, A. E., Walker-Caulfield, M. E., Ebel, M. E., & Brown, M. A. (2015). Cutting edge: c-Kit signaling differentially regulates type 2 innate lymphoid cell accumulation and susceptibility to central nervous system demyelination in male and female SJL mice. J Immunol, 194(12), 5609-5613. doi: 10.4049/jimmunol.1500068
16. Woodruff TK, Green S, Paller A, Schlosser BJ, Spring B, Castle M, Stock MC, Carnethon MR, Clark CT, Gerard E, Turek FW, Wisner KL, Wakschlag LS, Kibbe MR, Mendelson MA, Simon MA, Hansen NM, Kenton K, Garcia PM, Zee P, Ramsey-Goldman R, Sutton SH, Van Horn L. (2015) Sex-based biomedical research policy needs an implementation plan. Womens Health (Lond Engl),11(4):449-52.
17. Klein, S. L., Schiebinger, L., Stefanick, M. L., Cahill, L., Danska, J., de Vries, G. J., . . . Zucker, I. (2015). Opinion: Sex inclusion in basic research drives discovery. Proc Natl Acad Sci U S A, 112(17), 5257-5258. doi: 10.1073/pnas.1502843112
18. Woodruff, T. K., Kibbe, M. R., Paller, A. S., Turek, F. W., & Woolley, C. S. (2014). Commentary: "Leaning in" to support sex differences in basic science and clinical research. Endocrinology, 155(4), 1181-1183. doi: 10.1210/en.2014-1068
19. 60 Minutes: http://www.cbsnews.com/news/sex-matters-drugs-can-affect-sexes-differently/
20. Colbert Report: http://www.cc.com/video-clips/el90zp/the-colbert-report-co-ed-lab-rats
21. Schiebinger, L., Klinge, I., Sánchez de Madariaga, I., Schraudner, M., and Stefanick, M. (Eds.) (2011-2013). Gendered Innovations in Science, Health & Medicine, Engineering, and Environment (genderedinnovations.stanford.edu).
22. Gaskill, B. N., Rohr, S. A., Pajor, E. A., Lucas, J. R., Garner, J. P. (2009). Some Like it Hot: Mouse Temperature Preferences in Laboratory Housing. Applied Animal Behavioral Science, 116, 279-285.
23. Ritz, S. A., Antle, D. M., Cote, J., Deroy, K., Fraleigh, N., Messing, K., . . . Mergler, D. (2014). First steps for integrating sex and gender considerations into basic experimental biomedical research. FASEB J, 28(1), 4-13. doi: 10.1096/fj.13-233395
24. Sorge, R. E., Martin, L. J., Isbester, K. A., Sotocinal, S. G., Rosen, S., Tuttle, A. H., . . . Mogil, J. S. (2014). Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods, 11(6), 629-632. doi: 10.1038/nmeth.2935
___
Teresa K. Woodruff, Ph.D.
Vice Chair for Research
Department of Obstetrics and Gynecology
Northwestern University
 Most women get heartburn from time to time. It may come as a burning sensation in the chest, or a bitter taste in the back of the throat. Heartburn is one word people use to describe reflux. It happens when stomach contents come back upwards. Reflux is sometimes painless: You may have trouble swallowing or get a dry cough, perhaps some wheezing. Occasional reflux episodes are normal. Like millions of Americans, you can manage reflux by avoiding foods that don’t agree with you—things that are fatty, spicy or acidic—or by eating smaller meals. If reflux occurs less than once a week, you can usually cope by making lifestyle changes or using over-the-counter medications. “We all have a little reflux when we burp or belch,” says Dr. John Pandolfino of Northwestern University.
Most women get heartburn from time to time. It may come as a burning sensation in the chest, or a bitter taste in the back of the throat. Heartburn is one word people use to describe reflux. It happens when stomach contents come back upwards. Reflux is sometimes painless: You may have trouble swallowing or get a dry cough, perhaps some wheezing. Occasional reflux episodes are normal. Like millions of Americans, you can manage reflux by avoiding foods that don’t agree with you—things that are fatty, spicy or acidic—or by eating smaller meals. If reflux occurs less than once a week, you can usually cope by making lifestyle changes or using over-the-counter medications. “We all have a little reflux when we burp or belch,” says Dr. John Pandolfino of Northwestern University.
 Researchers have identified a key step in the establishment of a pregnancy. The discovery may shed light on fertility disorders and diseases of the uterus, including endometrial cancer. At the start of each menstrual cycle, levels of the hormone estrogen begin to rise, which causes the uterine lining to grow and thicken. When the ovary releases an egg, levels of another hormone, progesterone, increase. Higher progesterone levels put the brakes on the estrogen-driven growth of the uterine lining, allowing the lining to mature and egg implantation to take place. Because of this function, progesterone is sometimes given to women to treat infertility and prevent premature birth. However, it carries some unpleasant side effects. A greater understanding of how progesterone works could lead to better treatments. It could also shed light on disorders such as endometrial cancer and endometriosis, which is marked by uncontrolled growth of the uterine lining.
Researchers have identified a key step in the establishment of a pregnancy. The discovery may shed light on fertility disorders and diseases of the uterus, including endometrial cancer. At the start of each menstrual cycle, levels of the hormone estrogen begin to rise, which causes the uterine lining to grow and thicken. When the ovary releases an egg, levels of another hormone, progesterone, increase. Higher progesterone levels put the brakes on the estrogen-driven growth of the uterine lining, allowing the lining to mature and egg implantation to take place. Because of this function, progesterone is sometimes given to women to treat infertility and prevent premature birth. However, it carries some unpleasant side effects. A greater understanding of how progesterone works could lead to better treatments. It could also shed light on disorders such as endometrial cancer and endometriosis, which is marked by uncontrolled growth of the uterine lining.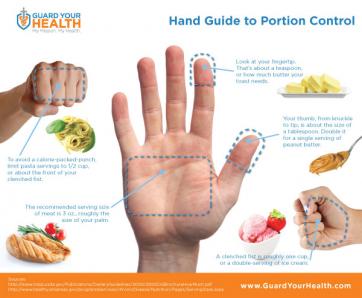 The holidays are officially over--and perhaps you have a New Year's resolution to lose weight? You're not alone! Losing weight is one of the most common New Year's resolutions, but many women struggle with the proper, healthy way to do this. Research has shown that portion control may be the most effective form of dieting when you take into account longevity and sustained weight loss and management. The reasonmay be that portion control is an easier behavioral target than planned exercise. Although increasing consumption of fruits and vegetables may be the easiest way to change behaviorally, it does not appear to be as effective in long term weight reduction. Okay, so all I have to do is eat a little less, and move a little more. Well, it’s not that simple. If you’ve tried portion control in the past, you might be rolling your eyes, because perhaps it was only a temporary fix. So how can we make portion control work for us? The important thing to understand is that portion sizes are often FAR LESS than we think they are. In fact, research has shown that Americans typically underestimate their caloric intake by as much as 25%. So if I think I’m only eating 1600 Calories (a typical weight loss goal), I might actually be eating 2,000.
The holidays are officially over--and perhaps you have a New Year's resolution to lose weight? You're not alone! Losing weight is one of the most common New Year's resolutions, but many women struggle with the proper, healthy way to do this. Research has shown that portion control may be the most effective form of dieting when you take into account longevity and sustained weight loss and management. The reasonmay be that portion control is an easier behavioral target than planned exercise. Although increasing consumption of fruits and vegetables may be the easiest way to change behaviorally, it does not appear to be as effective in long term weight reduction. Okay, so all I have to do is eat a little less, and move a little more. Well, it’s not that simple. If you’ve tried portion control in the past, you might be rolling your eyes, because perhaps it was only a temporary fix. So how can we make portion control work for us? The important thing to understand is that portion sizes are often FAR LESS than we think they are. In fact, research has shown that Americans typically underestimate their caloric intake by as much as 25%. So if I think I’m only eating 1600 Calories (a typical weight loss goal), I might actually be eating 2,000.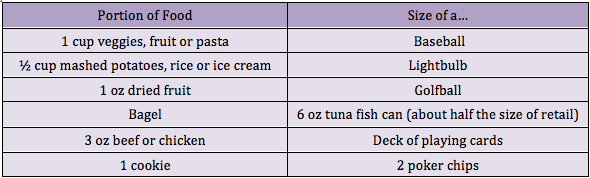
 Today the FDA announced an expansion of existing research projects to foster new studies related to advancing the science of women's health. The announcement of the
Today the FDA announced an expansion of existing research projects to foster new studies related to advancing the science of women's health. The announcement of the  Over the years the Women's Health Research Institute has written hundreds of blogs aimed at better informing our community on issues relating to women's health and sex-inclusion in science. As we learn more about sex differences, it is easy to understand why both men and women need to be included in clinical research. But why does the sex of a cell used in basic research matter? Cell lines and primary cells are often used by basic scientists in proof-of-concept experiments and when trying to figure out how biological mechanisms work. These early findings help provide valuable clues for developing new drugs, treatments and diagnostic models that eventually can be applied to humans. In the end, to truly advance sex based research, we must ensure that changes are made in partnership with all members of the scientific pipeline: the cell and animal suppliers, the bench scientist, the clinical scientist, the Institutional Review Boards who approve human study designs, the funding agencies, and the Journals who publish scientific outcomes. Including sex as a variable in basic and clinicial science will lead to better, more targeted discoveries that affect the health of all people!
Over the years the Women's Health Research Institute has written hundreds of blogs aimed at better informing our community on issues relating to women's health and sex-inclusion in science. As we learn more about sex differences, it is easy to understand why both men and women need to be included in clinical research. But why does the sex of a cell used in basic research matter? Cell lines and primary cells are often used by basic scientists in proof-of-concept experiments and when trying to figure out how biological mechanisms work. These early findings help provide valuable clues for developing new drugs, treatments and diagnostic models that eventually can be applied to humans. In the end, to truly advance sex based research, we must ensure that changes are made in partnership with all members of the scientific pipeline: the cell and animal suppliers, the bench scientist, the clinical scientist, the Institutional Review Boards who approve human study designs, the funding agencies, and the Journals who publish scientific outcomes. Including sex as a variable in basic and clinicial science will lead to better, more targeted discoveries that affect the health of all people! The Women's Health Research Institute wrote the following letter advocating for better parameters for sex-inclusion at the basic science level:
The Women's Health Research Institute wrote the following letter advocating for better parameters for sex-inclusion at the basic science level: Tis the season for sweet treats! We all know that eating sweet and starchy foods is not the best for our health, but did you know that these foods have been found to increase a woman’s risk of endometrial
Tis the season for sweet treats! We all know that eating sweet and starchy foods is not the best for our health, but did you know that these foods have been found to increase a woman’s risk of endometrial  Do you have questions about menopause? Are you ever curious about
Do you have questions about menopause? Are you ever curious about  Do you ever find yourself stressed during the holiday season? You're not alone! Research by
Do you ever find yourself stressed during the holiday season? You're not alone! Research by  Since the mid-19th century, researchers claimed they could identify the sex of an indivudual just by examining their disembodied brain. However, a new study out of Tel Aviv University disputes this. Indeed, all brians share characteristics, some more common in males, others more common in females, and other characteristics that are common in both of the sexes. While there are some general tendencies for each sex--for instance, men tend to have a larger amygdala--these differences are quite small and highly influenced by the environment. Essentially, throughout history, scientists have attempted to find the differences between male and female brains, when, in actuality, their similarieis are far greater in number.
Since the mid-19th century, researchers claimed they could identify the sex of an indivudual just by examining their disembodied brain. However, a new study out of Tel Aviv University disputes this. Indeed, all brians share characteristics, some more common in males, others more common in females, and other characteristics that are common in both of the sexes. While there are some general tendencies for each sex--for instance, men tend to have a larger amygdala--these differences are quite small and highly influenced by the environment. Essentially, throughout history, scientists have attempted to find the differences between male and female brains, when, in actuality, their similarieis are far greater in number.