Throughout my career, I have been a strong proponent of medical research through consumer advocacy and educational activities. But recently, I had the opportunity to actually participate as a research subject! As an enrollee in the Illinois Women's Health Registry, my health profile matched the criteria that a researcher was seeking for an osteoarthritis (OA) study. The project is called the MAK-3 Study and its purpose is to explore whether people with stronger hip muscles have a slower rate of OA progression and whether different factors of knee instability are related to knee OA progression.
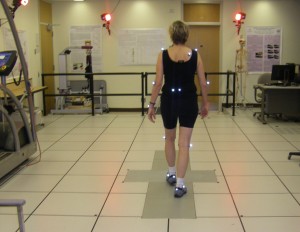
My camera flash picked up the reflector balls/markers taped to my joints --- could this be the new look in jewelry!!
My Registry profile included "knee pain" which flagged me as a potential match for this study. I gave permission for the Registry staff to give my name to the research coordinator for the study and I was selected! The study is being done at Northwestern University and it involves 2 or 3 visits for a variety of non-invasive evaluation procedures. They would be done at baseline (this past month) and repeated two years later. This is what they call an "observational" study. It will not directly benefit me, but it would help scientists better understand this condition and the factors that influence it. Since my family was riddled with osteoarthritis, I wanted to participate.
I chose to do the evaluations in three rather than two sessions because it fit my work schedule better. At the first session, I filled out numerous surveys about pain, my medications and my exercise habits. A physical therapist watched me walk down the hall several times to observe my "gait". I was then hooked up to equipment that measured muscle strength ( it looked like much of the equipment you see at your local gym, just with gauges to measure strength). They asked me to perform some typical knee and hip movements like leg lifts and recorded my muscle strength. After that a physician examined my legs and hips and reviewed my medication history. I was asked if I would provide an optional urine sample that would be stored to eventually be used to test any new biomarker tests for OA that may be developed. I was provided a diary where I recorded by physical activity for a week at home.
At the second session I met the team in a research lab that was equipped with sensors along the ceiling and a hollow floor that ran wires to a lot of computerized equipment. They taped small reflective balls to my joints and had me walk up and down the room many times while the sensors picked up the signals from these balls (see picture!) When I finished, the researcher showed me what the sensors were detecting. The computer screen showed a stick figure (me) with multicolored dots on my joints (representing the reflecting balls) moving across the screen. They were recording my alignment and gait. It was very interesting to see my stick figure move across the screen. Once they pulled off the sensor balls, they measured the speed of my normal walk by making me walk down a hallway and timing me.
A few weeks later I went to a different location where I had a special x-ray taken that included, on one film, my ankle, knee and hip joint to see how they lined up. This was followed by rather long MRIs on each of my knees. This was the hardest (but tolerable) part of the study, I needed to stay still for 40 minutes per knee. At least they gave me headphones and my head was outside the MRI machine where I watched a bird trying to get through the window. Throughout this experience the research coordinator explained what would happen and why it was done. Since I was going to physical therapy for my knee at the time, it was helpful to have her explain where they saw strengths and weakness in my hips and knees. It made me appreciate why the PT was having me do certain exercises on these related muscle groups.
Finally, the best part of this experience was knowing that I may actually be contributing to science, with little interruption (total 7- 8 hours) in my life. They will have me back in two years to repeat these tests and determine the status of my OA symptoms. By the way, I was given a stipend to cover my expenses like parking---and just enough to take a few girlfriends out for a nice lunch and get them to join the Illinois Women's Health Registry!
 In response to the call for more sex inclusion data in drug studies, the FDA has developed Drug Trials Snapshot a pilot project to provide information about the sex, age, race and ethnicity of clinical trial participants for a small group of recently approved drugs. In addition to information about who participates in the trial, each Snapshot also includes information on how the study was designed, results of the efficacy and safety studies and, if known, differences in efficacy and side effects among sex, race and age (referred to as subgroups).
In response to the call for more sex inclusion data in drug studies, the FDA has developed Drug Trials Snapshot a pilot project to provide information about the sex, age, race and ethnicity of clinical trial participants for a small group of recently approved drugs. In addition to information about who participates in the trial, each Snapshot also includes information on how the study was designed, results of the efficacy and safety studies and, if known, differences in efficacy and side effects among sex, race and age (referred to as subgroups).

 particular issue. Or when there is more data in a statistically significant pool that truly reflects our population diversity and allows us to determine differences that may exist among different races and ethnicities. This could lead to different approaches for certain populations. We'd like to think of research as finite but it is a constantly revolving cycle. One important example is the recent mammography guideline debate. Several years ago, mammography screening became mainstream: most women knew the guidelines, advocates worked to pass laws to assure that all women had access, and early studies showed that finding breast cancer early, in many cases, improved survival rates. Last November the U.S Preventive Task Force new mammography guidelines that were
particular issue. Or when there is more data in a statistically significant pool that truly reflects our population diversity and allows us to determine differences that may exist among different races and ethnicities. This could lead to different approaches for certain populations. We'd like to think of research as finite but it is a constantly revolving cycle. One important example is the recent mammography guideline debate. Several years ago, mammography screening became mainstream: most women knew the guidelines, advocates worked to pass laws to assure that all women had access, and early studies showed that finding breast cancer early, in many cases, improved survival rates. Last November the U.S Preventive Task Force new mammography guidelines that were