Our success as researchers is measured by our ability to translate our findings, according to the often-used phrase, from bench to bedside. In other words, if we can apply our basic science findings to clinical care, we have the ability to impact countless lives. This pipeline is a national priority, and in fact, many Academic Medical Centers have established programs to facilitate rapid clinical translation. However, equally as important, and perhaps less appreciated is the need to translate basic science findings into relevant policies that protect and influence the general public.
Reproductive science and medicine are greatly impacted by the environment. Trends in reproductive health demonstrate that reproductive function has declined since the mid-20th century in certain populations and locations [1]. Coincident with this decline in reproductive function is the large and ever-increasing number of natural and synthetic chemicals to which humans are exposed [2, 3]. Basic, clinical, and epidemiological research has demonstrated that exposure to certain compounds and contaminants, such as Endocrine Disrupting Chemicals (EDC), can have negative impacts on reproductive health. These compounds interfere with the production, transport, activity, and metabolism of natural hormones in the body. As we, as basic scientists and clinical researchers, understand the mechanisms by which these environmental exposures to such compounds affect developmental, reproductive, and neuroendocrine functions, we must also be able to inform and educate the implications of these specific reproductive health findings to the decision makers in Washington, DC. The question is: How?
In 2010, the Program on Reproductive Health and the Environment at the University of California, San Francisco developed the Reach the Decision Makers Fellowship with the exact intent of providing interested individuals and teams with the resources to advance science-based policy solutions. Specifically the Reach Program serves to provide individuals with a distinct interest in reproductive health and the environment, with mechanisms to interact with the United States Environmental Protection Agency (US-EPA). Over the past two years, the Reach program directed by Tracey Woodruff, PhD, MPH, an esteemed leader in the field, has trained over 75 individuals nationwide based on the principles of participatory democracy, social justice, and taking action to prevent harm (for more recent news about the Reach Program, check out the following blog written for the Physicians for Social Responsibility).
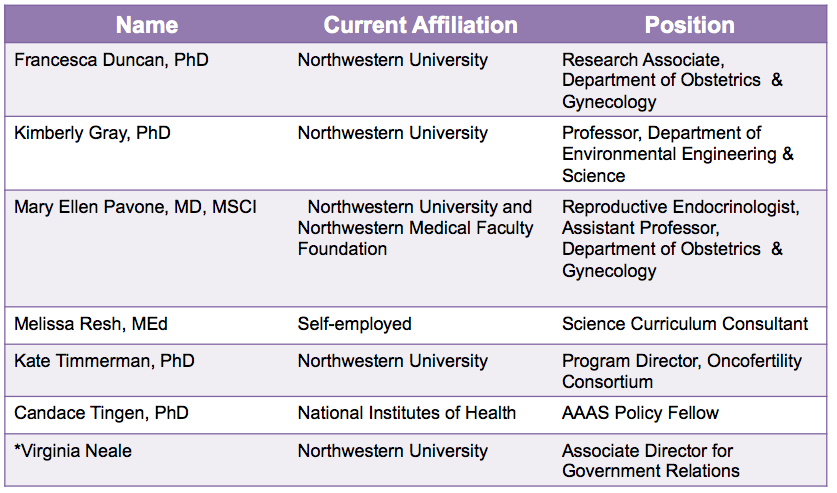
Table 1. Our interdisciplinary team
To take advantage of this unique program, we assembled an interdisciplinary team of six individuals committed to reproductive health and the environment (Table 1). Our team is comprised of professionals from academia, health care, government, and the community, and collectively we have experience in research, policy, advocacy, teaching, and communication (Table 2). Prior to joining the Reach Program, our team has worked together at Northwestern University and Northwestern Memorial Hospital in various settings including the Women’s Health Research Institute, the laboratory of Teresa K. Woodruff, PhD, the Oncofertility Consortium, the National Physicians Cooperative, the Oncofertility Saturday Academy, and the proposed Northwestern University Superfund Research Center in Reproductive Health Hazards. We joined the Reach Program with the goal of ensuring that the manner in which the US-EPA evaluates reproductive health and toxicity is in line with the current state of scientific knowledge.
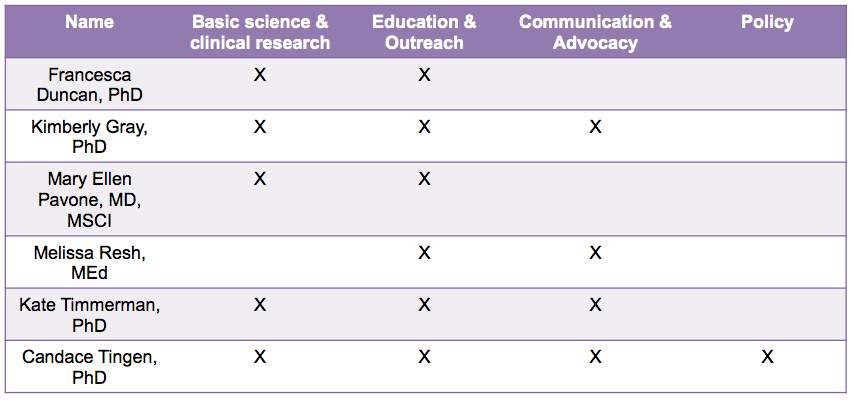
Table 2. Team backgrounds
As Reach Program fellows, we have participated in a rigorous training program to define a reasonable “ask” in relation to our goal, to perform research on the topic, and to learn how to identify the key decision makers within the US-EPA who will listen to our request and affect change. Over the past six months, we have engaged in a first trip to Washington, DC where we attended presentations from policy experts regarding the US-EPA hazard evaluation procedures and how scientists can inform the agency on emerging research regarding the effects of environmental toxins on reproductive health. Meetings at the US-EPA gave the team a greater understanding of the overall institution and current initiatives of the agency. We have also participated in nine webinars covering topics spanning from the effects of environmental toxins on reproductive health to identifying policies and policy makers at the US-EPA.
We also developed our policy project by systematically gaining an understanding of the US-EPA as an agency and the documents and guidelines that inform its staff. Members of the US-EPA helped us identify a principle document in reproductive health and the environment, the Guidelines for Reproductive Toxicity Risk Assessment. This document was written in 1996 and has not been revised since that time so our group decided to focus on some of the significant opportunities to improve upon the guidelines. Since 1996, the state of reproductive research has advanced and we identified three specific areas of research that could be prioritized during the updating of the Guidelines for Reproductive Toxicity Risk Assessment, as follows:
- While the Guidelines acknowledged the importance of non-reproductive consequences of an impaired reproductive system, such as osteoporosis and increased risk of stroke, they did not include these outcomes as endpoint measures for further research study.
- Model organisms are necessary for advancing research in reproductive and environmental health. In the Guidelines, the authors state that effects seen in one organism may be assumed to occur in another. While this is meant to be protective for unstudied species, it is also true that certain species are ideal to investigate different aspects of science and health. Thus, we encourage the study of multiple model organisms in reproductive health and the environment.
- Research advances over the past decade have shown that significant sex differences are seen in the way males and females respond to different drugs and environmental toxins. This warrants the need to include both sexes in reproductive research, a consideration that could strengthen the updated Guidelines.
Our team developed these ideas into a position statement to inform US-EPA staff and interested parties of the need to advance reproductive health and the environment. This project culminates tomorrow, Thursday, October 18, 2012 when the team will fly to Washington, D.C. to meet with Nica Louie (Environmental Health Scientist at the National Center for Environmental Research), Brenda Foos (Director, Regulatory Support and Science Policy Division, Office of Children's Health Protection), and Daniel Axelrad (Environmental Scientist, Office of Policy) at the US-EPA. We hope to gain a greater understanding of the procedures of the agency at these meetings and advocate for the need to update Guidelines for Reproductive Toxicity Risk Assessment.
Virginia Neale, the Associate Director of Government Relations for Northwestern University, will also join the team and bring her expertise in bridging academia and the government to the project. Neale also facilitated a meeting between team members and legislative assistants to the House of Representatives congresswoman Jan Schakowsky (D-IL), who resides over Northwestern University’s Evanston campus. As congressional requests to the US-EPA are often needed to gather teams of experts and update guidelines, we will ask Schakowsky’s office to make such a request to gather the National Academy of Sciences and revise the Guidelines for Reproductive Toxicity Risk Assessment.
The work done this week, and over the past six months, by this interdisciplinary group, will build the foundation for the team to continue communicating evidence-based reproductive health findings to the policy makers in Washington D.C. who have the ability to affect change on a federal level. The relationships we develop this week will be fostered in the coming months and years to ensure that reproductive health is promoted at the highest level within the EPA and advocate that US-EPA guidelines are updated to include the most recent advances in reproductive research
This blog was Contributed by Francesca Elizabeth Duncan, PhD and Kate Waimey Timmerman, PhD Read more about the team here in a Northwestern University press release.
1. Woodruff, T.J., J. Schwartz, and L.C. Giudice, Research agenda for environmental reproductive health in the 21st century. Journal of epidemiology and community health, 2010. 64(4): p. 307-10.
2. Sutton, P., L.C. Giudice, and T.J. Woodruff, Reproductive environmental health. Current opinion in obstetrics & gynecology, 2010. 22(6): p. 517-24.
3. Woodruff, T.J., et al., Proceedings of the Summit on Environmental Challenges to Reproductive Health and Fertility: executive summary. Fertility and sterility, 2008. 89(2 Suppl): p. e1-e20.
 Yesterday morning we awoke to a political landscape that seems jarred by the process of democracy, but ready to move forward as a nation. Three issues defined the outcome: the percent of women who chose democratic principles; the resounding losses by candidates who are antiquated in their thinking about pregnancy, in particular; and, the need to hold all of us accountable as citizens in the care of each other starting at the research bench to the bedside. I’m a reproductive scientist and direct the Women’s Health Research Institute at Northwestern University, so these issues are my issues and it is now time to look forward and identify actionable steps that moves our field forward.
Yesterday morning we awoke to a political landscape that seems jarred by the process of democracy, but ready to move forward as a nation. Three issues defined the outcome: the percent of women who chose democratic principles; the resounding losses by candidates who are antiquated in their thinking about pregnancy, in particular; and, the need to hold all of us accountable as citizens in the care of each other starting at the research bench to the bedside. I’m a reproductive scientist and direct the Women’s Health Research Institute at Northwestern University, so these issues are my issues and it is now time to look forward and identify actionable steps that moves our field forward.
 Ninety seven percent of online pharmacies don't follow U.S. pharmacy laws. If you buy from one of these online pharmacies, you run a high risk of receiving counterfeit or substandard drugs. You also put your personal and financial information at risk.
Ninety seven percent of online pharmacies don't follow U.S. pharmacy laws. If you buy from one of these online pharmacies, you run a high risk of receiving counterfeit or substandard drugs. You also put your personal and financial information at risk. Conditions that affect the brain can be more complicated in women compared to men, partly because of hormones and reproductive issues. Did you know:
Conditions that affect the brain can be more complicated in women compared to men, partly because of hormones and reproductive issues. Did you know: The breast cancer rate in the UK per 100,000 women in 2010 was practically double the rate in 1971. A number of UK scientists attribute this dramatic rise in the rates to the routine exposure to toxic chemicals, which are added to personal care, beauty, and household products. However, with the introduction of regular screening in 1987, the mortality rates have dramatically declined. Proving cause and effect of environmental exposure cause is difficult, time-consuming, and expensive but the interest is growing. These chemicals are getting into women’s bodies by applying them to the skin, by inhaling them, and by eating and drinking. The report from
The breast cancer rate in the UK per 100,000 women in 2010 was practically double the rate in 1971. A number of UK scientists attribute this dramatic rise in the rates to the routine exposure to toxic chemicals, which are added to personal care, beauty, and household products. However, with the introduction of regular screening in 1987, the mortality rates have dramatically declined. Proving cause and effect of environmental exposure cause is difficult, time-consuming, and expensive but the interest is growing. These chemicals are getting into women’s bodies by applying them to the skin, by inhaling them, and by eating and drinking. The report from  Oral medication for treating a type of incontinence in women is roughly as effective as Botox injections to the bladder, reported researchers who conducted a National Institutes of Health clinical trials network study, with each form of treatment having benefits and limitations.
Oral medication for treating a type of incontinence in women is roughly as effective as Botox injections to the bladder, reported researchers who conducted a National Institutes of Health clinical trials network study, with each form of treatment having benefits and limitations. A recent study reported that girls who were sexually abused often avoid cervical cancer screenings as adults. Not surprising, most of the girls who completed the study survey avoid the screening test not just because of embarrassment of being abused, but because of physical scars of abuse that would be seen by the screener. For an in-depth review of this issue and recommendations for health providers who provide these tests, we've included a
A recent study reported that girls who were sexually abused often avoid cervical cancer screenings as adults. Not surprising, most of the girls who completed the study survey avoid the screening test not just because of embarrassment of being abused, but because of physical scars of abuse that would be seen by the screener. For an in-depth review of this issue and recommendations for health providers who provide these tests, we've included a 

 Study Explores Psychosocial Implications Related to Relationships, Marriage and Childbearing
Study Explores Psychosocial Implications Related to Relationships, Marriage and Childbearing